��Ŀ����
��8�֣���֪A��B��C��D��E��F�dz��л�ѧ���������ʡ�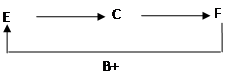
��1��ͨ��״���£�A��BΪ���塣A��ʹ�����ǵ�ľ����ȼ������B�������˹����ꡣ��AΪ______________��B_______________��[д��ѧʽ����ͬ]
��2�������£�C��DΪҺ�壬�Ҷ������Ԫ����ͬ����һ�������¿ɷ�����ӦD��C+A����CΪ____________��DΪ______________��
��3��E��F��������Ԫ����ɣ���B��C����ͼ��ʾ���ת������Ӧ��ϵ�����ַ�Ӧ������P��Ӧ��������ȥ������EΪ____________��FΪ_______________����ӦC��F�Ļ�ѧ����ʽΪ___________________________��
��8�֣�
��1��___O2___��__ CO2_ �� ��2��__H2O_��_H2O2_��
��3��CaCO3 ��Ca(OH)2��
��4��CaO+H2O=Ca(OH)2 �� ��2�֣�
����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
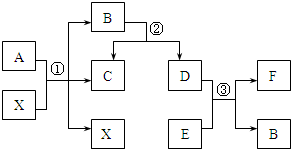 ��֪A��B��C��D��E��FΪ�������ʣ�����A��B������ͬ��Ԫ�أ�B��EΪ�����C��D��F��Ϊ���ʣ�����֮���ת���Ĺ�ϵ����ͼ��ʾ��ͼ�з�Ӧ��������ȥ����
��֪A��B��C��D��E��FΪ�������ʣ�����A��B������ͬ��Ԫ�أ�B��EΪ�����C��D��F��Ϊ���ʣ�����֮���ת���Ĺ�ϵ����ͼ��ʾ��ͼ�з�Ӧ��������ȥ���� ��2013?��������֪A��B��C��D�dz��л�ѧ���������ʣ�����֮����ת����ϵ��ͼ��ʾ������A������������ġ���ɫ��Դ����B�ڳ�������һ����ɫҺ�壬C��������𣮣����ַ�Ӧ�������ͷ�Ӧ����δ�����
��2013?��������֪A��B��C��D�dz��л�ѧ���������ʣ�����֮����ת����ϵ��ͼ��ʾ������A������������ġ���ɫ��Դ����B�ڳ�������һ����ɫҺ�壬C��������𣮣����ַ�Ӧ�������ͷ�Ӧ����δ�����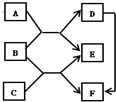 ��ͼ��֪A��B��C��D��E��FΪ���л�ѧ���������ʣ������������Ӧ��ת����ϵ��ͼ��
��ͼ��֪A��B��C��D��E��FΪ���л�ѧ���������ʣ������������Ӧ��ת����ϵ��ͼ��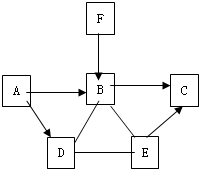 ��֪A��B��C��D��E��F�dz��л�ѧ�г������������ʣ�����֮����һ������������ͼ��ʾ��ת����ϵ��ͼ�С�������ʾ���ʼ����ת����ϵ����
��֪A��B��C��D��E��F�dz��л�ѧ�г������������ʣ�����֮����һ������������ͼ��ʾ��ת����ϵ��ͼ�С�������ʾ���ʼ����ת����ϵ����