��Ŀ����
��������̼������������ǵĻ�����
��1�����оٴ벻�ܴﵽ����̼��Ŀ�ĵ��� ������ĸ����
A.�ᳫʹ�ý��ܵ�
B.���úͿ���̫���ܡ����ܵ���Դ
C.ѡ���С������г�������������ͨ���ߵȷ�ʽ����
D.�㷺ʹ��һ���Կ��ӡ�һ�������ϴ�
��2��Ϊ���ٶ�����̼������������ŷţ���ѧ�Ҳ�ȡ�����ת������������������̼�������ڴ����ͼ���������ת��Ϊһ����Ҫ�Ļ���ԭ����ϩ��C2H4����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��1��D
��2��2CO2+6H2 C2H4+4H2O
C2H4+4H2O
��������1��A��ʹ�ý��ܵƿɽ�Լ��Դ��B�пɼ��ٻ�ʯȼ�ϵ�ʹ�ã�C��Ҳ�ɽ�ʡ��Դ����Щ���ɼ��ٶ�����̼���ŷţ��Ӷ��ﵽ����̼����Ŀ�ġ�D�й㷺ʹ��һ���Կ��ӻ����ĸ����ľ�ģ��ƻ�ɭ����Դ�������˶Զ�����̼�����գ����ܴﵽ����̼����Ŀ�ģ���������ʯ��Ϊԭ�������ģ�����������Ҳ�����ĸ������Դ����������Ķ�����̼���㷺ʹ��һ�������ϴ���Ҳ���ܴﵽ����̼����Ŀ�ġ�
��2��������֪����Ӧ��ΪCO2��H2��������ΪC2H4��H2O����Ӧ�����Ǵ����ͼ��ȣ��ݴ˿�д����Ӧ�Ļ�ѧ����ʽ��

 �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�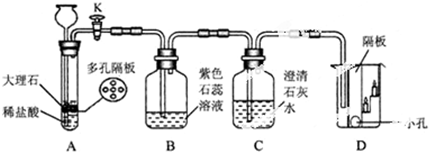
 2010��4��22���ǵ�41����������գ�����Ϊ����ϧ������Դת�䷢չ��ʽ��������̼�������������������ÿ������Ӧ������������Σ����оٴ벻������һ������ǣ�������
2010��4��22���ǵ�41����������գ�����Ϊ����ϧ������Դת�䷢չ��ʽ��������̼�������������������ÿ������Ӧ������������Σ����оٴ벻������һ������ǣ������� 2010��4��22���ǵ�41����������գ�����Ϊ����ϧ������Դת�䷢չ��ʽ��������̼�������������������ÿ������Ӧ������������Σ����оٴ벻������һ�������
2010��4��22���ǵ�41����������գ�����Ϊ����ϧ������Դת�䷢չ��ʽ��������̼�������������������ÿ������Ӧ������������Σ����оٴ벻������һ�������
