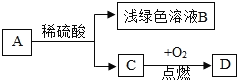
��Ŀ����
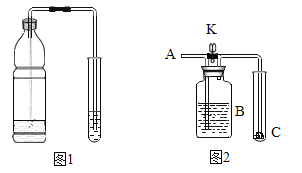
����Ŀ������ͼ��ʾװ�ý���ʵ�飬�о�ȼ�յ�������
��֪�������Ż��Ϊ40�棬�����Ż��Ϊ240�档
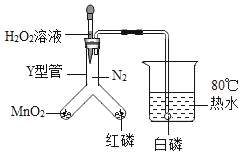
��1����H2O2��Һ��MnO2�Ӵ�ʱ��������Ӧ�Ļ�ѧ ����ʽΪ_____���÷�Ӧ����_____��Ӧ�����������Ӧ���ͣ�
��2����Y���м���H2O2��Һ�۲쵽���ܿڿ�ʼ��������ʱ���ձ��а��ײ�ȼ�գ�һ��ʱ�����ȼ�գ��������ܹ�֤���Ŀ�ȼ��ȼ�յ�������_____����֤����ȼ��ȼ�յ���һ�����������ݵ�������_____��
���𰸡�2H2O2![]() 2H2O+O2�� �ֽ� ��Ҫ�������Ӵ� �ձ��а���ȼ��ʱ��Y���к��ײ�ȼ��
2H2O+O2�� �ֽ� ��Ҫ�������Ӵ� �ձ��а���ȼ��ʱ��Y���к��ײ�ȼ��
��������
��1�����������ڶ������̵Ĵ�����������ˮ����������Ӧ�Ļ�ѧ����ʽΪ��2H2O2![]() 2H2O+O2�����÷�Ӧ������һ���������ʽ�����ϷֽⷴӦ�����������ڷֽⷴӦ��
2H2O+O2�����÷�Ӧ������һ���������ʽ�����ϷֽⷴӦ�����������ڷֽⷴӦ��
��2����Y���м���H2O2��Һ�۲쵽���ܿڿ�ʼ��������ʱ���ձ��а��ײ�ȼ�գ�һ��ʱ�����ȼ�գ�֤���Ŀ�ȼ��ȼ�յ���������Ҫ�������Ӵ����ձ��а���ȼ��ʱ��Y���к��ײ�ȼ�գ�˵����ȼ��ȼ�յ��������¶�Ҫ�ﵽ��ȼ����Ż�㡣

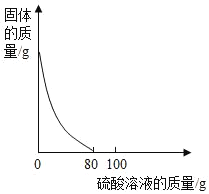
 ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�����Ŀ������ͼ������ȷ��ӳ��Ӧ�仯��ϵ����
|
|
|
|
A����һ��������AgNO3��Cu(NO3)2�Ļ����Һ�в��ϼ������� | B����Ũ����¶���ڿ����� | C. �ں��������£������͵�NaCl��Һ��������ˮ | D����һ�������ı���ʯ��ˮ�м�����ʯ�� |
A. A B. B C. C D. D