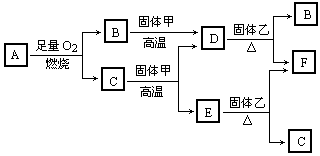
题目内容
根据要求书写化学方程式:(1)市售紫葡萄的表皮上常附有一种浅蓝色的斑点,它是为了防治葡萄霉菌而喷洒的农药波尔多液.波尔多液可由硫酸铜溶液与石灰水混合制得,配制时不能用铁制容器.其中石灰水可由生石灰与水反应得到,而生石灰可由煅烧石灰石得到.按下列要求写出以上叙述中涉及反应的化学方程式:
化合反应______;
分解反应______ CaO+CO2↑
【答案】分析:利用常见物质的化学性质,结合物质除杂和净化的要求,以及常见的反应类型解答此题.
解答:解:(1)由题意可知,涉及的化学反应为:化合反应 CaO+H2O═Ca(OH)2 分解反应 CaCO3═CaO+CO2↑ 置换反应 Fe+CuSO4═FeSO4+Cu 复分解反应 CuSO4+Ca(OH)2═Cu(OH)2↓+CaSO4
(2)除杂质的关键是生成气体或沉淀这种易于分离的物质,根据常见物质的性质可知:①Ca(OH)2+Na2CO3═CaCO3↓+2NaOH ②MgCl2+2NaOH═Mg(OH)2↓+2NaCl ③BaCO3+H2SO4═BaSO4↓+H2O+CO2↑
④Zn+CuCl2═ZnCl2+Cu
综上,故答案为:(1)CaO+H2O═Ca(OH)2 CaCO3═CaO+CO2↑ Fe+CuSO4═FeSO4+Cu CuSO4+Ca(OH)2═Cu(OH)2↓+CaSO4
(2)①Ca(OH)2+Na2CO3═CaCO3↓+2NaOH ②MgCl2+2NaOH═Mg(OH)2↓+2NaCl ③BaCO3+H2SO4═BaSO4↓+H2O+CO2↑ ④Zn+CuCl2═ZnCl2+Cu
点评:本题考查了常见物质的性质,化学方程式的书写,物质除杂和净化的要求,以及常见的反应类型,锻炼了学生分析问题解决问题的能力.
解答:解:(1)由题意可知,涉及的化学反应为:化合反应 CaO+H2O═Ca(OH)2 分解反应 CaCO3═CaO+CO2↑ 置换反应 Fe+CuSO4═FeSO4+Cu 复分解反应 CuSO4+Ca(OH)2═Cu(OH)2↓+CaSO4
(2)除杂质的关键是生成气体或沉淀这种易于分离的物质,根据常见物质的性质可知:①Ca(OH)2+Na2CO3═CaCO3↓+2NaOH ②MgCl2+2NaOH═Mg(OH)2↓+2NaCl ③BaCO3+H2SO4═BaSO4↓+H2O+CO2↑
④Zn+CuCl2═ZnCl2+Cu
综上,故答案为:(1)CaO+H2O═Ca(OH)2 CaCO3═CaO+CO2↑ Fe+CuSO4═FeSO4+Cu CuSO4+Ca(OH)2═Cu(OH)2↓+CaSO4
(2)①Ca(OH)2+Na2CO3═CaCO3↓+2NaOH ②MgCl2+2NaOH═Mg(OH)2↓+2NaCl ③BaCO3+H2SO4═BaSO4↓+H2O+CO2↑ ④Zn+CuCl2═ZnCl2+Cu
点评:本题考查了常见物质的性质,化学方程式的书写,物质除杂和净化的要求,以及常见的反应类型,锻炼了学生分析问题解决问题的能力.

练习册系列答案
相关题目