��Ŀ����
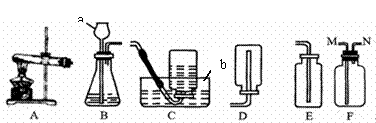 ʵ���ҳ�������װ������ȡ���壺
ʵ���ҳ�������װ������ȡ���壺
(1)д��ͼ���б�����������ƣ�a___________��b_____________��
(2)������غͶ�����������ȡ����ʱ����ѡ�õķ���װ��Ϊ ����дװ�ñ�ţ�������Ӧ�Ļ�ѧ����ʽΪ__________________________________________��
(3)��Eװ���ռ�������̼��������_________������ ����������Ƿ�Ϊ������̼������Fװ�����ռ�CO2������Ӧ��_________���ܿڽ��롣(ѡ�M����N��)
(4)��ͼ��ʵ������˫��ˮ��ȡ������������Ҫ���裬��Щ�������ȷ˳����(����ĸ��ţ���ͬ)_________�����в����������_________��
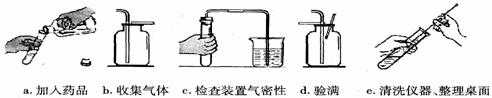
(1)����©����ˮ�ۣ�
(2) A��2 KClO3 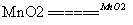 2 KCl + 3 O2��
2 KCl + 3 O2��
(3) ������̼������ܶȱȿ�������ʯ��ˮ��M
(4) cabde��d

��ϰ��ϵ�д�
�����Ŀ

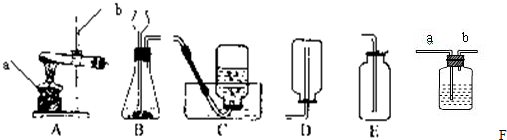

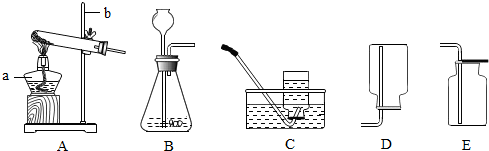
 ʵ���ҳ�������װ������ȡ������
ʵ���ҳ�������װ������ȡ������