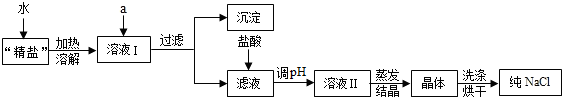
��Ŀ����
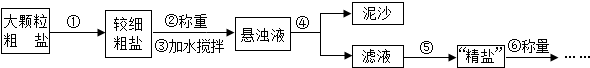
�ᴿ��������ɳ�Ĵ��Σ�һ�㾭���²������̣�
��1���������б����õ���һ��������
A���ƾ��� B����Ͳ C���ձ� D���Թ�
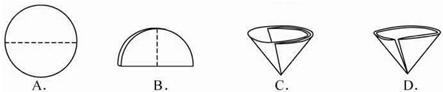
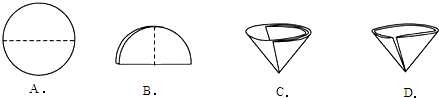
��2������������Ҫ��Բ����ֽ�۵�����������ͼʾ�в��ó��ֵ�������
��3���������г��õ����żܡ��ƾ��ơ�������������ǯ�⣬����Ҫ�õ�
A ���������Ͻ��� B �����ƶ��ƾ��Ƽ��� C �����ƶ����������
��4��ʵ������������õľ��Σ������㾫�ε��Ƶ��ʣ������Ƶ��ʽϵͣ������ԭ����
A��ʳ��û��ȫ���ܽ⼴���� B������ʱʳ�ηɽ�����
C�����������þ��κܳ�ʪ D��������մ�еľ���ûȫ��ת�Ƶ�����ֽ��
��5�����ؽᾧ���������ᴿ�������裻
��1���������б����õ���һ��������
C
C
������ţ���A���ƾ��� B����Ͳ C���ձ� D���Թ�
��2������������Ҫ��Բ����ֽ�۵�����������ͼʾ�в��ó��ֵ�������
D
D
������ţ�����3���������г��õ����żܡ��ƾ��ơ�������������ǯ�⣬����Ҫ�õ�
������
������
���������ò������������ʳ�ι���ɽ���Ϊ�������ٷɽ����ɲ�ȡ��ABC
ABC
�ȴ�ʩ��A ���������Ͻ��� B �����ƶ��ƾ��Ƽ��� C �����ƶ����������
��4��ʵ������������õľ��Σ������㾫�ε��Ƶ��ʣ������Ƶ��ʽϵͣ������ԭ����
ABD
ABD
������ţ���A��ʳ��û��ȫ���ܽ⼴���� B������ʱʳ�ηɽ�����
C�����������þ��κܳ�ʪ D��������մ�еľ���ûȫ��ת�Ƶ�����ֽ��
��5�����ؽᾧ���������ᴿ�������裻
�����þ����ܽ⣬Ȼ����ˣ���������Һ���������ľ���
�����þ����ܽ⣬Ȼ����ˣ���������Һ���������ľ���
��
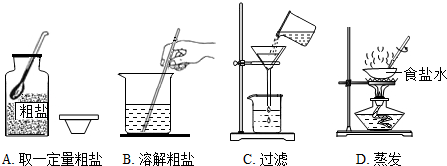
��������1����������������װ�õ����ý��з�����
��2������ͼʾ�е���ֽ���۵��������з�����
��3�������Ȼ�����Һ������������Ҫ���������з�����
���� ����������Ⱦ���������з�����
��4�����Ը������������ı���ʽ���з��������ε��������˻��ߴ��ε��������ˣ�
��2������ͼʾ�е���ֽ���۵��������з�����
��3�������Ȼ�����Һ������������Ҫ���������з�����
���� ����������Ⱦ���������з�����
��4�����Ը������������ı���ʽ���з��������ε��������˻��ߴ��ε��������ˣ�
����⣺��1���ɲ������̿���֪��������Ϊ���˲��������Է�����������������֪���ձ��DZز����ٵģ���ѡC��
��2��A��B��C ����ֽ����ȷ���۵�������D�е���ֽ���ӿڴ����ַ�϶�����Բ����ܳ�������״������ѡD��
��3��������Ϊ��ʳ����Һ�������������Գ�������������������Ҫʢ��Һ��ļ���װ��������Ϊ��ʹҺ�����Ⱦ��ȶ���ֹҺ�εķɽ������Լȿ��Խ��裬Ҳ����ͨ���ƶ��ƾ��ƻ����ƶ���������ʹ��Һ���ȸ����ȣ�
��4�����εIJ��ʵ�˵�����ᴿʱ����ʧ���Ȼ��ƣ�
A��ʳ��û����ȫ�ܽ⣬��ʹ���ε��������٣���A��ȷ��
B��ʳ�ηɽ��˵����˾��εļ��٣���B��ȷ��
C�����κܳ������˾��ε���������ʹ������������C����
D���������еľ���û��ȫ��ת�Ƶ�����ֽ�ϣ���ʹ���ε�������С����D��ȷ��
��ѡABD��
��5�����ؽᾧ���������ᴿ��Ȼ��Ҫ�����ܽ⣬���ˡ���������һ���̣����䲽��Ϊ�������þ����ܽ⣬Ȼ����ˣ���������Һ���������õ����壮
�ʴ�Ϊ����1��C��
��2��D��
��3��A B C��
��4��A B D��
��5�������þ����ܽ⣬Ȼ����ˣ���������Һ���������ľ��壮
��2��A��B��C ����ֽ����ȷ���۵�������D�е���ֽ���ӿڴ����ַ�϶�����Բ����ܳ�������״������ѡD��
��3��������Ϊ��ʳ����Һ�������������Գ�������������������Ҫʢ��Һ��ļ���װ��������Ϊ��ʹҺ�����Ⱦ��ȶ���ֹҺ�εķɽ������Լȿ��Խ��裬Ҳ����ͨ���ƶ��ƾ��ƻ����ƶ���������ʹ��Һ���ȸ����ȣ�
��4�����εIJ��ʵ�˵�����ᴿʱ����ʧ���Ȼ��ƣ�
A��ʳ��û����ȫ�ܽ⣬��ʹ���ε��������٣���A��ȷ��
B��ʳ�ηɽ��˵����˾��εļ��٣���B��ȷ��
C�����κܳ������˾��ε���������ʹ������������C����
D���������еľ���û��ȫ��ת�Ƶ�����ֽ�ϣ���ʹ���ε�������С����D��ȷ��
��ѡABD��
��5�����ؽᾧ���������ᴿ��Ȼ��Ҫ�����ܽ⣬���ˡ���������һ���̣����䲽��Ϊ�������þ����ܽ⣬Ȼ����ˣ���������Һ���������õ����壮
�ʴ�Ϊ����1��C��
��2��D��
��3��A B C��
��4��A B D��
��5�������þ����ܽ⣬Ȼ����ˣ���������Һ���������ľ��壮
������������Ҫ�����Ȼ���������ᴿ�IJ��輰ע�����ѧ��̽��ʵ�����ݴ��������������ķ������ɣ�

��ϰ��ϵ�д�
 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�
�����Ŀ