��Ŀ����
ijУ��ѧʵ����ϣ�ͬѧ���û�ѧ����̽��һ�ָ���Ʒ�����ĺ�����ͬѧ�ǽ�11.4g����Ʒ��20.0gϡ���ᣨ��������������Ϊ120.0g�ձ��У��ڻ�ѧ��Ӧ�����ж��ձ������е�ʣ����������Ĵγ�������¼���±���| ��Ӧʱ�� | T0 | T1 | T2 | T3 |
| �ձ���ҩƷ����/g | 151.4 | 151.3 | 151.0 | 151.0 |
��1����Ӧ�в���������������
��2���ֵ����ͷ�Ϊ��
| ��̼�� | ��̼�� | ��̼�� |
| ��̼��0.03%-0.3% | ��̼��0.3%-0.6% | ��̼��0.6%-2% |
��3��ij�ֳ���ұ��1000t���ֲָģ��躬������80%�ij�������ٶ֣�
��������1����ͼʾ���ݿ��Կ�����T2��T3ʱ��ʣ������������ȣ�˵��T2ʱ��Ӧ����ȫ�����岻�ٲ��������������غ㶨�ɣ���Ӧ�в�������������=��Ӧǰ���������ܺ�-T2��Ӧ�����������ܺͣ�
��2�������������ᷴӦ�Ļ�ѧ����ʽ�������������������г�����ʽ���Ϳɼ��������Ʒ�к������������������������Ʒ�к�̼������������Ʒ����-����Ʒ�к�������������Ȼ���������������ʽ�Ϳɼ��������Ʒ�ĺ�̼��������ͼ�����ݣ������ж����ָ������������͵ĸ֣�
��3�����ݣ�2���м�����ĸ���Ʒ�ĺ�̼�����ó�����Ʒ�ĺ�������1-����Ʒ�ĺ�̼��������1000t���ֵĺ����������ұ��1000t���ֲָĺ���������������������������������������������������������ٳ���80%���������������������
��2�������������ᷴӦ�Ļ�ѧ����ʽ�������������������г�����ʽ���Ϳɼ��������Ʒ�к������������������������Ʒ�к�̼������������Ʒ����-����Ʒ�к�������������Ȼ���������������ʽ�Ϳɼ��������Ʒ�ĺ�̼��������ͼ�����ݣ������ж����ָ������������͵ĸ֣�
��3�����ݣ�2���м�����ĸ���Ʒ�ĺ�̼�����ó�����Ʒ�ĺ�������1-����Ʒ�ĺ�̼��������1000t���ֵĺ����������ұ��1000t���ֲָĺ���������������������������������������������������������ٳ���80%���������������������
����⣺��1����Ӧ�в�������������=11.4g+20.0g+120.0g-151.0g=0.4g���ʴ�Ϊ��0.4��
��2�������Ʒ�к���������Ϊx��
Fe+2HCl�TFeCl2+H2��
56 2
x 0.4g
��
=
��
��֮�ã�x=11.2g��
����Ʒ�еĺ�̼��=
��100%=1.75%��
����ͼ�����ݿ�֪�����ָ����ڸ�̼�֣�
��3��1000t����1-1.75%����70%��80%=1754.5t��
����Ҫ���ֳ�����ʯ1754.5t��
��2�������Ʒ�к���������Ϊx��
Fe+2HCl�TFeCl2+H2��
56 2
x 0.4g
��
| 56 |
| 2 |
| x |
| 0.4g |
��֮�ã�x=11.2g��
����Ʒ�еĺ�̼��=
| 11.4g-11.2g |
| 11.4g |
����ͼ�����ݿ�֪�����ָ����ڸ�̼�֣�
��3��1000t����1-1.75%����70%��80%=1754.5t��
����Ҫ���ֳ�����ʯ1754.5t��
������������Ҫ����ѧ�����û�ѧ����ʽ���м��������������Ĺؼ��Ǹ��������غ㶨��������������������Ϊ����ͻ�ƿڣ����µ�����Ϳ�ӭ�ж��⣮

��ϰ��ϵ�д�
 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
�����Ŀ
 ijУ��ѧ������ȤС���ͬѧ���о���ѧϰ����չʾ��һ������ͼ��ʾʵ��װ�ã�����ÿ����ѧ��Ӧ����ȫ����������Ʒ�е����ʲ��μӷ�Ӧ�����������ϣ�������Ũ�������ʱ���ȷ������·�Ӧ��H2C2O4
ijУ��ѧ������ȤС���ͬѧ���о���ѧϰ����չʾ��һ������ͼ��ʾʵ��װ�ã�����ÿ����ѧ��Ӧ����ȫ����������Ʒ�е����ʲ��μӷ�Ӧ�����������ϣ�������Ũ�������ʱ���ȷ������·�Ӧ��H2C2O4
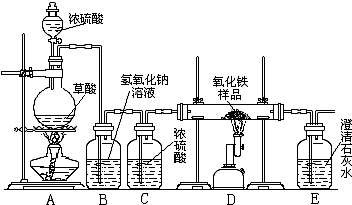
 CO��+CO2��+H2O
CO��+CO2��+H2O
