��Ŀ����
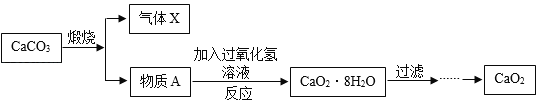
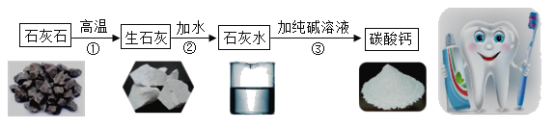
����Ŀ������̼��ƣ��ֳƳ���̼��ƣ����ߴ��ȵ�̼��ƣ����ܶ�С���ȸߡ�Ħ��ϵ��С�������������ԭ��֮һ�������ڸߵ���dzɫ��Ʒ�ϡ���ҵ��ͨ����ʯ��ʯ����Ҫ�ɷ�ΪCaCO3��Ϊԭ�ϣ�����ͼ��ʾ�������̽��иߴ���̼��Ƶ���ȡ���ᴿ��
����������Ϣ���Իش��������⣺
��ʯ��ʯ����;�dz��㷺�������й�˵����ȷ����_____������ѡ��
�ٿ�������ȡ������̼ �ڿ�������ȡ��ʯ�� �ۿ��������첣��
�ܿ���������������ˮ �ݿɼ���ú̿��������� ��������¯����������
A �٢ۢݢ� B �ڢܢݢ� C �٢ڢܢݢ� D ����ȫ������
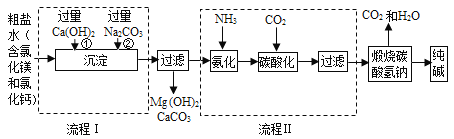
�������������漰�������У��������������������_____��
���������̵IJ�����У�������ѡ������һ���Լ����ó������Ʊ����ߴ��ȵ�̼��ƣ��䷴Ӧԭ���Ļ�ѧ����ʽΪ_____��
���Ʊ��ߴ���̼���Ҫ������ʯ��ʯ��CaCO3������ʯ����ʯ��ˮ��CaCO3����ת�����̣���Ŀ����_____��
��Сӱͬѧ��ȡ��25.0gijʯ��ʯ��Ʒ������ͼ���̽�����ģ��ʵ��̽�������ڲ��������ʯ��ˮ��μ���������������Ϊ10%��Na2CO3��Һ���ù����в������������������Na2CO3��Һ��������ϵ��ͼ��ʾ���Լ��㣺
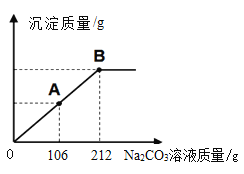
����ʵ���У�Сӱ�����Ƶ�����̼��Ƶ�������_____�������ȷ��0.1g��
���𰸡�C ��ʯ�� CO2+Ca(OH)2=CaCO3��+H2O ��ȥʯ��ʯ�е����� 21.2g
��������
�� �������첣������̼���ƣ���ѡC��
����ʯ������ˮ��Ӧ�����������ƣ������������漰�������У����������������������ʯ�ң�
�����������������̼��ӦҲ������̼��ƣ��䷴Ӧԭ���Ļ�ѧ����ʽΪCO2+Ca(OH)2=CaCO3��+H2O��
����ҵ�ϵ�ʯ��ʯ�����������ʣ��Ʊ��ߴ���̼���Ҫ������ʯ��ʯ��CaCO3������ʯ����ʯ��ˮ��CaCO3����ת�����̣���Ŀ���dz�ȥʯ��ʯ�е����ʡ�
��5��������̼��Ƶ�����Ϊx

x=21.2g��

 ��У����ϵ�д�
��У����ϵ�д�