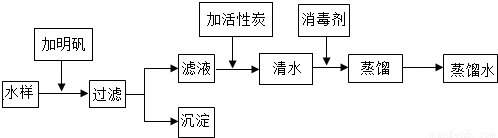
��Ŀ����
��2011?���գ�����װ������ʵ������CO2���Ʊ������������飬����ռ�һƿ�����CO2����ش��������⣺
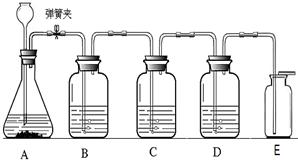
��1����ʵ������ȡ������̼ҩƷѡ���̽��ʵ�飬��¼���£�
����ȡ���ռ��ĽǶȷ�����һ��ѡ���
��2����C��D�е�Һ�����������±���
��3��B�з�Ӧ�Ļ�ѧ����ʽ��
��4��C�з�Ӧ�Ļ�ѧ����ʽ��
��5����Ӧ�����н����ɼйرգ���A�п�����������
��6��E�ռ�����˵��������̼���е�����������

��1����ʵ������ȡ������̼ҩƷѡ���̽��ʵ�飬��¼���£�
| ��� | ҩƷ | ʵ������ |
| �� | ̼���Ʒ�ĩ��ϡ���� | �����������ʺܿ� |
| �� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
| �� | ��״ʯ��ʯ��ϡ���� | ���������������� |
��
��
������ţ���ҩƷ����������Ӧ�Ļ�ѧ����ʽΪCaCO3+2HCl�TCaCl2+H2O+CO2��
CaCO3+2HCl�TCaCl2+H2O+CO2��
����2����C��D�е�Һ�����������±���
| B���Σ� | C��� | D���ᣩ | |
| Һ������ | ̼��������Һ | ����ʯ��ˮ ����ʯ��ˮ | Ũ���� Ũ���� |
NaHCO3+HCl�TNaCl+H2O+CO2��
NaHCO3+HCl�TNaCl+H2O+CO2��
����4��C�з�Ӧ�Ļ�ѧ����ʽ��
CO2+Ca��OH��2�TCaCO3��+H2O
CO2+Ca��OH��2�TCaCO3��+H2O
����5����Ӧ�����н����ɼйرգ���A�п�����������
A����ƿ��Һ���½�������©����Һ������
A����ƿ��Һ���½�������©����Һ������
����6��E�ռ�����˵��������̼���е�����������
������̼���ܶȱȿ�����
������̼���ܶȱȿ�����
����������1����ʵ������ȡ������̼ҩƷѡ��ѡ��ҩƷ������ʵ�����ܳ������ȶ��ķ�����ѧ��Ӧ�����������ٶ����У������ռ������û�ѧ����ʽ��ʾ�䷴Ӧԭ����
��2��������������װ������ʵ������CO2���Ʊ������������飬����ռ�һƿ�����CO2��Ҫ��ѡ����顢����CO2�����Һ��ҩƷ��
��3��ѡ��̼��������Һ�����Ȼ������壬д��������Ӧ�Ļ�ѧ����ʽ��
��4�����������̼����ķ������������ʯ��ˮ��ͨ������̼������ǣ�д���䷴Ӧ�Ļ�ѧ����ʽ��
��5����Ӧ�����н����ɼйرգ���ƿ�ڲ������������ų�����ѹ������A�п�����������A����ƿ��Һ���½�������©����Һ��������
��6��E�ռ��������ö�����̼�ܶȱȿ�������������ʣ�
��2��������������װ������ʵ������CO2���Ʊ������������飬����ռ�һƿ�����CO2��Ҫ��ѡ����顢����CO2�����Һ��ҩƷ��
��3��ѡ��̼��������Һ�����Ȼ������壬д��������Ӧ�Ļ�ѧ����ʽ��
��4�����������̼����ķ������������ʯ��ˮ��ͨ������̼������ǣ�д���䷴Ӧ�Ļ�ѧ����ʽ��
��5����Ӧ�����н����ɼйرգ���ƿ�ڲ������������ų�����ѹ������A�п�����������A����ƿ��Һ���½�������©����Һ��������
��6��E�ռ��������ö�����̼�ܶȱȿ�������������ʣ�
����⣺��1��ʵ������ȡ������̼ѡ��ҩƷʱ��ע�ؾ���ʵ�����ܳ������ȶ��ķ�����ѧ��Ӧ�����������ٶ����У������ռ���
�ʴ�Ϊ���ۣ� ��������Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2��
��2����Cװ���Ǽ��������̼����ļ�Һ���ʱ����� ����ʯ��ˮ����Dװ���Ǹ��������̼�������Һ���ʱ�����Ũ���ᣮ
��3����Bװ���Ǿ����Ȼ����������ҺNaHCO3���ʷ�Ӧ�Ļ�ѧ����ʽΪ��NaHCO3+HCl�TNaCl+H2O+CO2����
��4����Cװ���Ǽ��������̼����ļ�Һ�����䷴Ӧ�ķ���ʽΪ��CO2+Ca��OH��2�TCaCO3��+H2O
��5����Aװ�õķ�Ӧ�����н����ɼйرգ���ƿ�ڲ������������ų�����ѹ������A�оͻ������ƿ��Һ���½�������©����Һ��������
��6����Eװ�������ſ������ռ�������̼����˵�������ܶȱȿ�����
�ʴ�Ϊ����1���ۡ�CaCO3+2HCl�TCaCl2+H2O+CO2��
��2��
��3��NaHCO3+HCl�TNaCl+H2O+CO2��
��4��CO2+Ca��OH��2�TCaCO3��+H2O
��5��A����ƿ��Һ���½�������©����Һ������
��6��������̼���ܶȱȿ�����
�ʴ�Ϊ���ۣ� ��������Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2��
��2����Cװ���Ǽ��������̼����ļ�Һ���ʱ����� ����ʯ��ˮ����Dװ���Ǹ��������̼�������Һ���ʱ�����Ũ���ᣮ
��3����Bװ���Ǿ����Ȼ����������ҺNaHCO3���ʷ�Ӧ�Ļ�ѧ����ʽΪ��NaHCO3+HCl�TNaCl+H2O+CO2����
��4����Cװ���Ǽ��������̼����ļ�Һ�����䷴Ӧ�ķ���ʽΪ��CO2+Ca��OH��2�TCaCO3��+H2O
��5����Aװ�õķ�Ӧ�����н����ɼйرգ���ƿ�ڲ������������ų�����ѹ������A�оͻ������ƿ��Һ���½�������©����Һ��������
��6����Eװ�������ſ������ռ�������̼����˵�������ܶȱȿ�����
�ʴ�Ϊ����1���ۡ�CaCO3+2HCl�TCaCl2+H2O+CO2��
��2��
| B���Σ� | C��� | D���ᣩ | |
| Һ������ | ̼��������Һ | ����ʯ��ˮ | Ũ���� |
��4��CO2+Ca��OH��2�TCaCO3��+H2O
��5��A����ƿ��Һ���½�������©����Һ������
��6��������̼���ܶȱȿ�����
���������⿼����ʵ������ȡ������̼�����ҩƷѡ��̽������Ӧԭ�����侻�������顢�����ҩƷѡ��̽�����ռ�������֪ʶ��Ŀ��飬�漰֪ʶ��㣬̽������࣬�ۺ���ǿ��ʵ�Ƕ���ѧ����һ�����⣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��2011?���գ���һ�������£���һ���ܱ������ڷ���ij��Ӧ����÷�Ӧ�����и����ʵ��������±���ʾ������˵������ȷ���ǣ�������
|
��2011?���գ�����װ������ʵ������CO2���Ʊ������������飬����ռ�һƿ�����CO2����ش��������⣺
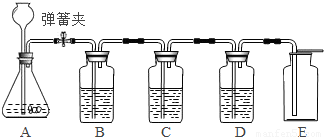
��1����ʵ������ȡ������̼ҩƷѡ���̽��ʵ�飬��¼���£�
����ȡ���ռ��ĽǶȷ�����һ��ѡ���______������ţ���ҩƷ����������Ӧ�Ļ�ѧ����ʽΪ______��
��2����C��D�е�Һ�����������±���
��3��B�з�Ӧ�Ļ�ѧ����ʽ��______��
��4��C�з�Ӧ�Ļ�ѧ����ʽ��______��
��5����Ӧ�����н����ɼйرգ���A�п�����������______��
��6��E�ռ�����˵��������̼���е�����������______��

��1����ʵ������ȡ������̼ҩƷѡ���̽��ʵ�飬��¼���£�
| ��� | ҩƷ | ʵ������ |
| �� | ̼���Ʒ�ĩ��ϡ���� | �����������ʺܿ� |
| �� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
| �� | ��״ʯ��ʯ��ϡ���� | ���������������� |
��2����C��D�е�Һ�����������±���
| B���Σ� | C��� | D���ᣩ | |
| Һ������ | ̼��������Һ | ______ | ______ |
��4��C�з�Ӧ�Ļ�ѧ����ʽ��______��
��5����Ӧ�����н����ɼйرգ���A�п�����������______��
��6��E�ռ�����˵��������̼���е�����������______��