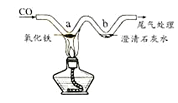
��Ŀ����
����Ŀ����ͼΪ����硱��Ƭ��Ʒ��ǩͼ������ݱ�ǩ���й���Ϣ������и��⡣

(1)��Ҫ�ɷ�̼�����__________��Ԫ����ɡ�
(2)̼����и�Ԫ�ص�ԭ�Ӹ�����Ϊ__________��
(3)ÿƬ��Ƭ�����ٺ���Ԫ�ص�����Ϊ__________g��
(4)С��ͬѧΪ�ⶨ����̼��Ƶĺ�����ע�Ƿ���ʵ����ȡ��10Ƭ��Ƭ����������С�ձ��У��ټ���50gϡ���ᣬ��T0��T3ʱ��Σ���÷�Ӧʣ����������仯���£�
�����ɵĶ�����̼��������__________��
��ͨ�������жϸø�Ƭ��̼��Ƶĺ�����ע�Ƿ���ʵ__________��
ʱ �� | T0 | T1 | T2 | T3 |
��Ӧʣ��������(g) | 70 | 65 | 63��4 | 63��4 |
���𰸡� �� Ca��C��O=1��1��3 0.6g 6.6g ��Ƭ��̼��Ƶĺ�����ע��ʵ
�����������⿼���˸��ݻ�ѧʽ�ͻ�ѧ����ʽ���������ݱ��������ݵõ����ɵĶ�����̼�����ǹؼ�����
��1����̼��ƵĻ�ѧʽ��֪��������̼���⡢������Ԫ����ɵģ�
(2)̼����и�Ԫ�ص�ԭ�Ӹ�����Ca��C��O=1��1��3��
��3��ÿƬ��Ƭ�����ٺ���Ԫ�ص�����Ϊ��1.5g��![]() ��100%=0.6g��
��100%=0.6g��
��4���ٸ��������غ㶨�ɣ����ɵĶ�����̼������Ϊ��70-63.4=6.6g��
�ڽ⣺��10Ƭ��Ʒ��̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
X 6.6g
![]() ��
��![]() x=15g��
x=15g��
ÿƬ��̼��Ƶ�����Ϊ�� ![]() =1.5g����Ƭ��̼��Ƶĺ�����ע��ʵ��
=1.5g����Ƭ��̼��Ƶĺ�����ע��ʵ��