��Ŀ����
��7�֣�����������ֻ���Ǻ�Al2O3��Fe2O3������Al�Ĺ����������£���ش��������⣺
���������ϡ���1����������Al2O3���Ժ�NaOH��Һ��Ӧ����Ӧ�Ļ�ѧ����ʽΪ��
Al2O3 + 2NaOH ="=" 2NaAlO2 + 2H2O��2��Fe2O3����NaOH��Һ��Ӧ��
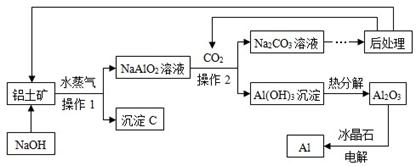
��1����ҵ�����У�Ҫ��������ϸĥԤ������Ŀ���� ��
��2������C�Ļ�ѧʽ�� ��
��3������1������2�������� ��ʵ������ɴ˲���ʱ����Ҫ�IJ����������Dz��������ձ��� ��
��4���˹����У���ѭ��ʹ�õ�������CO2��H2O�� �����ѧʽ��
��5�����Al2O3�Ļ�ѧ����ʽΪ ��
��6��NaAlO2��Һ��ͨ��CO2��Ӧ�Ļ�ѧ����ʽ�� ��
���������ϡ���1����������Al2O3���Ժ�NaOH��Һ��Ӧ����Ӧ�Ļ�ѧ����ʽΪ��
Al2O3 + 2NaOH ="=" 2NaAlO2 + 2H2O��2��Fe2O3����NaOH��Һ��Ӧ��

��1����ҵ�����У�Ҫ��������ϸĥԤ������Ŀ���� ��
��2������C�Ļ�ѧʽ�� ��
��3������1������2�������� ��ʵ������ɴ˲���ʱ����Ҫ�IJ����������Dz��������ձ��� ��
��4���˹����У���ѭ��ʹ�õ�������CO2��H2O�� �����ѧʽ��
��5�����Al2O3�Ļ�ѧ����ʽΪ ��
��6��NaAlO2��Һ��ͨ��CO2��Ӧ�Ļ�ѧ����ʽ�� ��
��1�����ӷ�Ӧ��Ӵ������ʹ��Ӧ��ֽ��У��ش���伴�ɵ÷֣���2��Fe2O3
��3������ ©�� ��4��NaOH ��5��2Al2O3 ="===" 4Al + 3O2��
(6) 2NaAlO2 + CO2 + 3H2O ="=" Na2CO3 + 2Al(OH)3��
��3������ ©�� ��4��NaOH ��5��2Al2O3 ="===" 4Al + 3O2��
(6) 2NaAlO2 + CO2 + 3H2O ="=" Na2CO3 + 2Al(OH)3��
�����������1����ҵ�����У�Ҫ��������ϸĥԤ������Ŀ�������ӷ�Ӧ��Ӵ������ʹ��Ӧ��ֽ��У�
��2������������Al2O3���Ժ�NaOH��Һ��Ӧ������������Ӧ���ʳ���C�Ļ�ѧʽ��Fe2O3��
��3������1������2�������ǹ��ˣ�ʵ������ɴ˲���ʱ����Ҫ�IJ����������Dz��������ձ���©����
��4�������й���ͼ��֪���˹����У���ѭ��ʹ�õ�������CO2��H2O��NaOH��
��5�����Al2O3�Ļ�ѧ����ʽΪ2Al2O3
 4Al + 3O2����
4Al + 3O2������6��NaAlO2��Һ��ͨ��CO2��Ӧ�Ļ�ѧ����ʽ��2NaAlO2 + CO2 + 3H2O ="=" Na2CO3 + 2Al(OH)3��
�������ƶ����ʣ�Ҫ�����й����ʵ����ʣ�ͨ����صķ�Ӧ���̣�����ѧ�ƶϣ�
��д��ѧ����ʽҪ��ѭ����ʵ�������غ㶨������ԭ��ע�⻯ѧʽҪ��ȷ����Ҫ���Ƿ�Ӧ������������߳������š�
���˿��Գ�ȥҺ���еĹ��岻���

��ϰ��ϵ�д�
 ����ѧ����ϵ�д�
����ѧ����ϵ�д�
�����Ŀ

 CO��+ H2O��
CO��+ H2O��