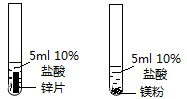
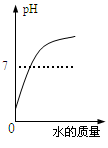
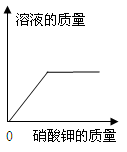
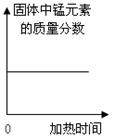
��Ŀ����
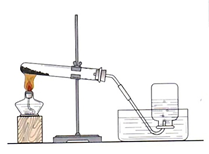
��10�֣���������ͼ��ʾ��ʵ��װ��ͼ����Ҫ��ش��й����⣺
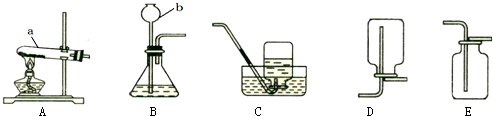
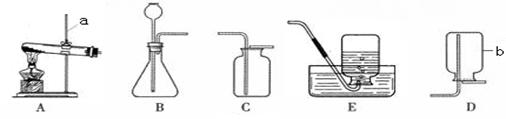
��1��д��ͼ�д��б�����������ƣ�a ��b ��
��2����Bװ����ȡ����ʱ���������Ļ�ѧ��Ӧ����ʽΪ ��
��3��ʵ�����ø��������ȡ���ռ������������Ӧѡ��װ�� ������ĸ�������������ռ����ķ����ǣ� ��
��4�������dz��л�ѧ������Ҫ��ʵ���ʵ��װ�á�
�� Aʵ����߲����ܢ� �в����������� ��?
��Bʵ���������Ϩ��˵��������̼���е������� ��
д��B�з�Ӧ�Ļ�ѧ����ʽ ��

��1��д��ͼ�д��б�����������ƣ�a ��b ��
��2����Bװ����ȡ����ʱ���������Ļ�ѧ��Ӧ����ʽΪ ��
��3��ʵ�����ø��������ȡ���ռ������������Ӧѡ��װ�� ������ĸ�������������ռ����ķ����ǣ� ��
��4�������dz��л�ѧ������Ҫ��ʵ���ʵ��װ�á�

�� Aʵ����߲����ܢ� �в����������� ��?
��Bʵ���������Ϩ��˵��������̼���е������� ��
д��B�з�Ӧ�Ļ�ѧ����ʽ ��
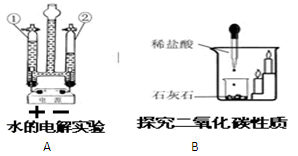
��1��a �Թ� ��b ����©������2�� 2H2O2MnO22H2O+O2�� ����3�� AE�� �Ѵ����ǵ�ľ������ƿ�ڣ�ľ����ȼ�����������۲��Ƿ�ȼ������4��Z�� ���� ��?�ڲ�֧��ȼ�գ�����ܶȺͲ���ȼ���۷֣��� CaCO3+2HCl==CaCl2+H2O+CO2��.
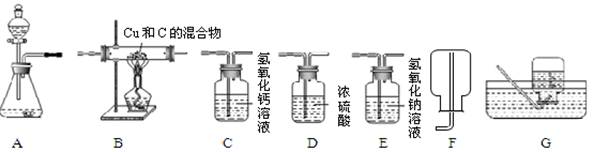
�����������1��ʵ���ҳ������������ƣ�
��2����Bװ����ȡ������Ϊ���塢Һ�峣���ͣ���д��ѧʽӦ��ע�⻯ѧʽ����ƽ����������ͷ��
��3�����ø�������������ǹ�����ȣ�װ����A����Ϊ�����ܶȱȿ������Ҳ�������ˮ�������ռ������������E�����������ķ�����
��4������ˮ����Ľ��ۼ�����̼��������ᷴӦ�����ɶ�����̼�Լ�������̼�����ʷ������ɽ��

��ϰ��ϵ�д�
 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
�����Ŀ