��Ŀ����
 ��2012?��������ģ��2011��9�£�ij���౻�غ����°���������������������ߵĽ��ǣ�����ͬѧ�ǿ�չ�˶�����ɷּ����õ�̽���������������ϵ�֪��
��2012?��������ģ��2011��9�£�ij���౻�غ����°���������������������ߵĽ��ǣ�����ͬѧ�ǿ�չ�˶�����ɷּ����õ�̽���������������ϵ�֪����1���ܶ�Ʒ��������С����������������еġ�������ָ
B
B
A�������� B����Ԫ�� C����ԭ��
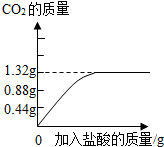
��2������ȥ����Ҫ��������Ħ�����ã�ijƷ�������е�Ħ������̼��ƣ�Ϊ�˼��鲢�ⶨ������̼��Ƶ�����������ͬѧ��ȡ��10g���࣬��������ϡ���Ტ���裮ʵ������м�¼�����������γ���ͼ���ߣ���Ʒ��������̼��Ƶ���������Ϊ���٣�
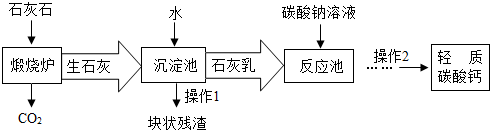
��3����������Ħ����������̼��ƿ���ʯ��ʯ���Ʊ�����ҵ����Ҫ�����������£�
�١�����¯���з�����Ӧ�Ļ�ѧ����ʽ�ǣ�
CaCO3
CaO+CO2��
| ||
CaCO3
CaO+CO2��
���ڡ���Ӧ�ء�����Ҫ������Ӧ�Ļ�ѧ����ʽ�ǣ�
| ||
Na2CO3+Ca��OH��2=CaCO3��+2NaOH
Na2CO3+Ca��OH��2=CaCO3��+2NaOH
���ڡ������ء�����ʯ����ˮ��ַ�Ӧ��ɵõ������dz�ϸС����ʯ�ҽ�����ʯ�ҽ�Ϊ
����Һ
����Һ
�����
�����
��ѡ�����Һ������Һ��������Һ�����������˽�����������������CO2�����̼������Һ�������������ŵ���
�ɽ��������ɱ��ͽ��ܼ���
�ɽ��������ɱ��ͽ��ܼ���
��С���������Ϻ��֪��
a��������̼����ͨ������������Һ�������·�Ӧ��
CO2+Ca��OH��2=CaCO3��+H2O��CaCO3+H2O+CO2=Ca��HCO3��2��
b��̼���������ˮ�����ֽ⣺Ca��HCO3��2
| ||
Ϊ�˷�ֹ���ø÷��Ƶõ�����̼����л���Ca��HCO3��2������2�б���Ҫ���е�һ��������
�������������������Ϊֹ
�������������������Ϊֹ
����������1��������������Ԫ����ɵģ������������������еġ�������ָ��Ԫ�ؽ��
��2������ͼʾ�ж�����̼����������ѧ����ʽ����̼��Ƶ��������������̼��Ƶ�����������
��3����̼��Ƹ����������������ƺͶ�����̼�Ͷ�����̼���������Ʒ�Ӧ�����������Ƴ�����ˮ��������д��ѧ����ʽ�IJ��裺д���䡢ע���ȣ���ȷ��д��ѧ����ʽ���ɣ�
����ʯ�ҽ�Ϊ����Һ��
��CO2�����̼������Һ���ɽ��������ɱ��ͽ��ܼ��ţ�����̼��������Ȳ��ȶ��ֽ�����̼��ƺ�ˮ���
��2������ͼʾ�ж�����̼����������ѧ����ʽ����̼��Ƶ��������������̼��Ƶ�����������
��3����̼��Ƹ����������������ƺͶ�����̼�Ͷ�����̼���������Ʒ�Ӧ�����������Ƴ�����ˮ��������д��ѧ����ʽ�IJ��裺д���䡢ע���ȣ���ȷ��д��ѧ����ʽ���ɣ�
����ʯ�ҽ�Ϊ����Һ��
��CO2�����̼������Һ���ɽ��������ɱ��ͽ��ܼ��ţ�����̼��������Ȳ��ȶ��ֽ�����̼��ƺ�ˮ���
����⣺��1����������Ԫ����ɵģ������������������еġ�������ָ��Ԫ�أ���ѡB��
��2�����Ʒ��������̼��Ƶ�����ΪX
2HCl+CaCO3=CaCl2+CO2��+H2O
100 44
X 1.32g
100��44=X��1.32g ��� X=3g
��Ʒ��������̼��Ƶ���������Ϊ��3g10g��100%=30%��
�𣺸�Ʒ��������̼��Ƶ���������Ϊ30%��
��3���ٸ�����д��ѧ����ʽ�IJ��裺
����¯���з�����Ӧ�Ļ�ѧ����ʽ�ǣ�CaCO3
CaO+CO2����
�ڡ���Ӧ�ء�����Ҫ������Ӧ�Ļ�ѧ����ʽ�ǣ�Na2CO3+Ca��OH��2=CaCO3��+2NaOH�� ����ʯ�ҽ�Ϊ����Һ��
��CO2�����̼������Һ���ɽ��������ɱ��ͽ��ܼ��ţ�
����̼��������Ȳ��ȶ��ֽ�����̼��ƺ�ˮ������2�б���Ҫ���е�һ�������ǣ��������������������Ϊֹ��
�ʴ�Ϊ����1��B��
��2�����Ʒ��������̼��Ƶ�����ΪX
2HCl+CaCO3=CaCl2+CO2��+H2O
100 44
X 1.32g
100��44=X��1.32g ��� X=3g
��Ʒ��������̼��Ƶ���������Ϊ��3g10g��100%=30%��
�𣺸�Ʒ��������̼��Ƶ���������Ϊ30%��
��3����CaCO3
CaO+CO2����Na2CO3+Ca��OH��2=CaCO3��+2NaOH��
������Һ��
�ۿɽ��������ɱ��ͽ��ܼ��� ������������Ҳ�ɣ��� �������������������Ϊֹ��
��2�����Ʒ��������̼��Ƶ�����ΪX
2HCl+CaCO3=CaCl2+CO2��+H2O
100 44
X 1.32g
100��44=X��1.32g ��� X=3g
��Ʒ��������̼��Ƶ���������Ϊ��3g10g��100%=30%��
�𣺸�Ʒ��������̼��Ƶ���������Ϊ30%��
��3���ٸ�����д��ѧ����ʽ�IJ��裺
����¯���з�����Ӧ�Ļ�ѧ����ʽ�ǣ�CaCO3
| ||
�ڡ���Ӧ�ء�����Ҫ������Ӧ�Ļ�ѧ����ʽ�ǣ�Na2CO3+Ca��OH��2=CaCO3��+2NaOH�� ����ʯ�ҽ�Ϊ����Һ��
��CO2�����̼������Һ���ɽ��������ɱ��ͽ��ܼ��ţ�
����̼��������Ȳ��ȶ��ֽ�����̼��ƺ�ˮ������2�б���Ҫ���е�һ�������ǣ��������������������Ϊֹ��
�ʴ�Ϊ����1��B��
��2�����Ʒ��������̼��Ƶ�����ΪX
2HCl+CaCO3=CaCl2+CO2��+H2O
100 44
X 1.32g
100��44=X��1.32g ��� X=3g
��Ʒ��������̼��Ƶ���������Ϊ��3g10g��100%=30%��
�𣺸�Ʒ��������̼��Ƶ���������Ϊ30%��
��3����CaCO3
| ||
������Һ��
�ۿɽ��������ɱ��ͽ��ܼ��� ������������Ҳ�ɣ��� �������������������Ϊֹ��
�����������ijƷ�������������ѧ�Ļ�ѧ֪ʶ�����ʵ���ɡ�̼��ƺ�ϡ����ķ�Ӧ��������̼���������Ƶķ�Ӧ�����ݻ�ѧ����ʽ�ļ�������ʵĴ��Ƚ����˿��飬Ҫ����������Щ֪ʶ����д��ѧ����ʽһ��Ҫȷ����

��ϰ��ϵ�д�
�����Ŀ
 ��2012?��������ģ��ר��ָ������ͯ���������ķ�ʽ֬���Ὣ��Ӱ�����巢������ͼ�����뵰������������Ϳ��ܻ����һ�ַ�ʽ֬���ᣬ�仯ѧʽ��C18H34O2���Ը�����˵����ȷ���ǣ�������
��2012?��������ģ��ר��ָ������ͯ���������ķ�ʽ֬���Ὣ��Ӱ�����巢������ͼ�����뵰������������Ϳ��ܻ����һ�ַ�ʽ֬���ᣬ�仯ѧʽ��C18H34O2���Ը�����˵����ȷ���ǣ�������