��Ŀ����
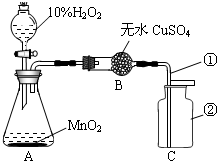 ˫��ˮ������������Ư�ȷ��棬�ڶ������̵������£���Ѹ�ٷֽ�ų�������Ԭ��ʦ�ͻ�ѧС���ͬѧ�ǰ���ͼװ��ʵ�飮��ش�
˫��ˮ������������Ư�ȷ��棬�ڶ������̵������£���Ѹ�ٷֽ�ų�������Ԭ��ʦ�ͻ�ѧС���ͬѧ�ǰ���ͼװ��ʵ�飮��ش���1��װ���б�������������ǣ���
��2����ƿ�ڷ�����Ӧ�Ļ�ѧ����ʽ��
����ø�������Ʊ���������ѧ����ʽ��
��3����ʵ����ʹ����100g10%��H2O2��Һ����H2O2��Һ�к�����H2O2
��4����ɫ����ˮ����ͭ��ˮ�������������Ԥ�Ȿʵ������У���ˮ����ͭ�Ƿ�������
��5�����������ſ������ռ�������ԭ����
��ȷ��
��6��֤��C���������ռ����ķ�����
��������1��ͬѧ�ǶԳ��û�ѧ�������˽��ʶ�������ֻ�кܺõ���ʶ���ǣ���ʵ����ܵ���Ӧ�֣�
��2������������ȡ�����Ļ�ѧ����ʽ��ע��д��Ҫ��飬һ��Ҫ��ƽ��
��3�������й������������������⣺100��10%=10�ˣ����Ը�H2O2��Һ�к�����H2O2Ϊ10�ˣ�
��4��˫��ˮѸ�ٷֽ�ų�������Ϊ���ȷ�Ӧ������ˮ�������ɣ�������ˮ����ͭ�������
��5����Ϊ�������ܶȴ��ڿ����ģ����������ſ������ռ�������
��6�����������������������������ǵ�ľ�����ڼ���ƿ�ڸ�ȼ��
��2������������ȡ�����Ļ�ѧ����ʽ��ע��д��Ҫ��飬һ��Ҫ��ƽ��
��3�������й������������������⣺100��10%=10�ˣ����Ը�H2O2��Һ�к�����H2O2Ϊ10�ˣ�
��4��˫��ˮѸ�ٷֽ�ų�������Ϊ���ȷ�Ӧ������ˮ�������ɣ�������ˮ����ͭ�������
��5����Ϊ�������ܶȴ��ڿ����ģ����������ſ������ռ�������
��6�����������������������������ǵ�ľ�����ڼ���ƿ�ڸ�ȼ��
����⣺��1����ǿ�Գ��û�ѧ�������˽��ʶ����������ܡ�����ƿ
��2������������ȡ�����Ļ�ѧ����ʽ��ע��д��Ҫ��飬һ��Ҫ��ƽ���������䷽����
��3�������й������������������⣺100��10%=10�ˣ�
��4��˫��ˮѸ�ٷֽ�ų�������Ϊ���ȷ�Ӧ������ˮ�������ɣ�������ˮ����ͭ�������
��5����Ϊ�������ܶȴ��ڿ����ģ����������ſ������ռ����������ܲ���λ����ȷ��
��6�����������������������������ǵ�ľ�����ڼ���ƿ�ڸ�ȼ��
�ʴ�Ϊ����1���ٵ��� �ڼ���ƿ ��2��2H2O2�T2H2O+O2�� 2KMnO4�TK2MnO4+MnO2+O2�� ��3��10��4���� ��5�������ܶȱȿ����� �ǣ���ȷ�� ��6���������ǵ�ľ�����ڼ���ƿ�ڸ�ȼ
��2������������ȡ�����Ļ�ѧ����ʽ��ע��д��Ҫ��飬һ��Ҫ��ƽ���������䷽����
��3�������й������������������⣺100��10%=10�ˣ�
��4��˫��ˮѸ�ٷֽ�ų�������Ϊ���ȷ�Ӧ������ˮ�������ɣ�������ˮ����ͭ�������
��5����Ϊ�������ܶȴ��ڿ����ģ����������ſ������ռ����������ܲ���λ����ȷ��
��6�����������������������������ǵ�ľ�����ڼ���ƿ�ڸ�ȼ��
�ʴ�Ϊ����1���ٵ��� �ڼ���ƿ ��2��2H2O2�T2H2O+O2�� 2KMnO4�TK2MnO4+MnO2+O2�� ��3��10��4���� ��5�������ܶȱȿ����� �ǣ���ȷ�� ��6���������ǵ�ľ�����ڼ���ƿ�ڸ�ȼ
�������������ڿ�����������ȡ˼·��������й������������������⣮

��ϰ��ϵ�д�
 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д�
�����Ŀ
 ˫��ˮ������������Ư�ȣ�����MnO2����ڳ����¼���Ѹ�ٷֽ�ų�������ʵ���ҿ�˫��ˮ��ȡ���������÷�Һ©�����������ġ����������ء�������ʱ����ƿ�еμ�˫��ˮ�����Իش��������⣺
˫��ˮ������������Ư�ȣ�����MnO2����ڳ����¼���Ѹ�ٷֽ�ų�������ʵ���ҿ�˫��ˮ��ȡ���������÷�Һ©�����������ġ����������ء�������ʱ����ƿ�еμ�˫��ˮ�����Իش��������⣺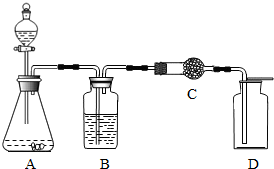 ˫��ˮ������������Ư�ȣ����ǹ������⣨H2O2����ˮ��Һ������������30%����Һ�Զ�������Ϊ������Ѹ�ٷֽ�������� �����Ƹ�����ִ������������ɲ�����ͼװ�ã�װ��A�У���ƿ��ʢ���Ƕ������̣���Һ©����ʢ����30%�Ĺ���������Һ��
˫��ˮ������������Ư�ȣ����ǹ������⣨H2O2����ˮ��Һ������������30%����Һ�Զ�������Ϊ������Ѹ�ٷֽ�������� �����Ƹ�����ִ������������ɲ�����ͼװ�ã�װ��A�У���ƿ��ʢ���Ƕ������̣���Һ©����ʢ����30%�Ĺ���������Һ�� ˫��ˮ������������Ư�ȷ��棬���ǹ������⣨H2O2������Һ����ʵ���������H2O2��Һ��ȡ������MnO2���������������ķ�Ӧ�ǣ�2H2O2
˫��ˮ������������Ư�ȷ��棬���ǹ������⣨H2O2������Һ����ʵ���������H2O2��Һ��ȡ������MnO2���������������ķ�Ӧ�ǣ�2H2O2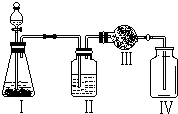 ˫��ˮ������������Ư�ȣ����ǹ������⣨H2O2����ˮ��Һ������������30%����Һ�Զ�������Ϊ��������Ѹ�ٷֽ�ų�������������Ӧ�Ļ�ѧ����ʽΪ��
˫��ˮ������������Ư�ȣ����ǹ������⣨H2O2����ˮ��Һ������������30%����Һ�Զ�������Ϊ��������Ѹ�ٷֽ�ų�������������Ӧ�Ļ�ѧ����ʽΪ��