��Ŀ����
����Ŀ������ѧ�������������ԡ��������ԡ�ͼ�����Եȡ�
(1)�û�ѧ���ű�ʾ��
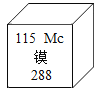
��þԪ��_________�� ��2��������_________��
����������_________�� ������ȱ��_________Ԫ�ػ�����Ͳ�
(2)��������ĸ�������
A.̼������ B. Ư�� C. ��ʯ�� D.����
��_________������ʳƷ����� ��_________���������Ƹ��ķ��ͷ�
��_________����������ˮ�������� ��__________��������ȡ������֬���ܼ�
(3)д�����з�Ӧ�Ļ�ѧ����ʽ
������������ȼ�գ�___________ ��
���Ȼ�����������ƻ�ϼ��ȣ�___________ ��
��̼����������������Һ��Ӧ��___________ ��
��������������Һ��Ӧ��___________ ��
���𰸡� Mg 2Al3+ FeSO4 Ca C A B D 3Fe + 2O2 ![]() Fe3O4�� 2NH4Cl+ Ca(OH)2
Fe3O4�� 2NH4Cl+ Ca(OH)2 ![]() CaCl2��2H2O��2NH3���� Na2CO3 + Ca(OH)2 == CaCO3��+ 2NaOH�� Fe+ 2AgNO3 == Fe (NO3)2 + 2Ag��
CaCl2��2H2O��2NH3���� Na2CO3 + Ca(OH)2 == CaCO3��+ 2NaOH�� Fe+ 2AgNO3 == Fe (NO3)2 + 2Ag��
�����������⿼���˳�����ѧ�������д�ͳ������ʵ���;����дʱע��淶��
(1) ��þԪ�ط�����Mg�������ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ������ʾ��������ӣ����������ӷ���ǰ������Ӧ�����֣�1�������Ӵ�3����λ�ĸ���ɣ� 2�������ӿɱ�ʾΪ��2Al3+����������������Ԫ����+2�ۣ��������-2�ۣ���ѧʽΪ��FeSO4��������ȱ�ٸ�Ԫ�ػ�����Ͳ�����Ԫ�ط���Ϊ��Ca��
(2) ����ʯ����������е�ˮ��Ӧ�����������ƣ�������ʳƷ�����������C����̼������������۷���ʱ�������������ʷ�Ӧ�����������Ƹ��ķ��ͷ�������A����Ư����ɱ������������������ˮ��������������B�����������ܽ���֬����������ȡ������֬���ܼ�������D��
(3)������������ȼ��������������������ѧ����ʽ����3Fe + 2O2 ![]() Fe3O4�����Ȼ�����������ƻ�ϼ��������Ȼ��ơ�������ˮ����ѧ����ʽ����2NH4Cl+ Ca(OH)2
Fe3O4�����Ȼ�����������ƻ�ϼ��������Ȼ��ơ�������ˮ����ѧ����ʽ����2NH4Cl+ Ca(OH)2 ![]() CaCl2��2H2O��2NH3������̼����������������Һ��Ӧ�����������ƺ�̼��ƣ���ѧ����ʽ����Na2CO3 + Ca(OH)2 == CaCO3��+ 2NaOH����������������Һ��Ӧ��������������ͭ����ѧ����ʽ����Fe+ 2AgNO3 == Fe (NO3)2 + 2Ag��
CaCl2��2H2O��2NH3������̼����������������Һ��Ӧ�����������ƺ�̼��ƣ���ѧ����ʽ����Na2CO3 + Ca(OH)2 == CaCO3��+ 2NaOH����������������Һ��Ӧ��������������ͭ����ѧ����ʽ����Fe+ 2AgNO3 == Fe (NO3)2 + 2Ag��