��Ŀ����
������Ŀ���й���һ�Һ���ĸ���������š���2012��9��25����ʽ�������ۣ������й��������ۣ���ĸ������������õIJ���Ҫ��ܸߣ��ر��Ƿ��мװ��õĸ�Ҫ�ߣ�ǿ���������ں�DZͧ�����й����������ϵ�������ȷ����( )
A. ����һ�ַǽ�������
B. ����һ�ֺϽ���Ӳ�ȱ���úϽ��н����ɷֵ��ʵ�Ӳ�ȸ�С
C. ����������������һ�������е�����
D. ���������ֻ��������ϵĽ�����һ�������ϳɵľ��н������Ե�����
�����ҹ���������ʳ����ʷ�ƾá�������õ�һ��ȼ���ǹ���ƾ���ij��ѧ��ȤС���ͬѧ�ԡ�����ƾ��������˺��棬����ɷֽ���̽��������ش��������⡣
���������ϣ�
a������ƾ�Ҳ����Ϊ���ƾ��顰�����ȼ�Ͽ顣����ƾ������ǹ���״̬�ľƾ����ǽ��ƾ���Ӳ֬����������ư�һ���������Ȼ���Ƴɡ�
b���ƾ��Ļ�ѧʽΪC2H5OH��
c���Ȼ������Ȼ�����Һ�������ԡ�
d��BaCl2+Na2CO3��BaCO3��+2NaCl ���ɵ�BaCO3Ϊ��ɫ����
��������⣩
��1���ƾ��Ļ�ѧʽ��NaOH��ȣ����С�OH������ô�ƾ���ˮ��Һ�Dz����Լ��ԣ�
��2������ƾ��е����������Ƿ���ʼ����ʵij̶���Σ�
��ʵ��̽��1���ƾ���ˮ��Һ�Dz����Լ���
ͬѧ��ȡ�����ƾ���Һ���Թ��У��μ���ɫʯ����Һ��δ�۲쵽��ɫʯ���Ϊ��ɫ��˵���ƾ���Һ_____����ԡ����ԡ������ԡ�
��ʵ��̽��2������ƾ��е����������Ƿ���ʼ����ʵij̶����
�ٹ���ƾ��е����������Ƿ���ʡ�
ͬѧ����ȡ��������ƾ����ձ��У���������ˮ�ܽ��μ�������ϡ���ᣬ�۲쵽_____����˵�����������ѱ��ʡ���д�����������ڿ����б��ʵĻ�ѧ����ʽ_____��
��Ϊ��һ��ȷ���������Ƶı��ʳ̶ȣ��������̽����
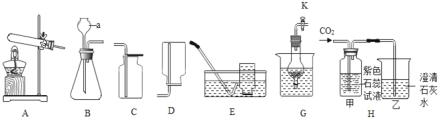
����ͬѧȡ�ձ��ϲ���Һ����֧�Թ��У�����ͼ��ʾ����ʵ�顣
ʵ�鷽�� |
|
|
ʵ������ | ��Һ��� | ����_____ |
ʵ����� | ��Һ������������ | ��Һ����̼���� |
����ͬѧ��Ϊ����ʵ�鲻��֤����Һ��һ�����������ƣ�������_____��������ȡ�ձ����ϲ���Һ���������Ȼ�����Һ����ַ�Ӧ���ã�ȡ�ϲ���Һ���μӷ�̪��Һ����̪��Һ��졣
����˼����������ʵ���м������Ȼ�����Һ��Ŀ����_____��
��ʵ�����]С��ͬѧ�������ۣ�һ����Ϊ�ù���ƾ��е��������Ʋ��ֱ��ʡ�