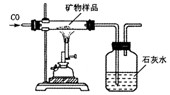
��Ŀ����
 ��֪ij����X�����������Ϣ����������Ҫ����Ļ�ѧ�ɷ���X2O3��������Ҫͨ���Ȼ�ԭ��ұ�����ɣ������������λ�ڽ���֮�ף�
��֪ij����X�����������Ϣ����������Ҫ����Ļ�ѧ�ɷ���X2O3��������Ҫͨ���Ȼ�ԭ��ұ�����ɣ������������λ�ڽ���֮�ף���1���ݴ��ƶ�X��
A���� B���� C���� D��ͭ
��2���ڸ�¯���ú�X2O3�Ŀ���ұ���ý�����ԭ����
��3���绯ѧ��ʴ�ǽ�����ʴ����Ҫԭ�����ֻ����Բ�ͬ�Ľ����ڳ�ʪ�Ļ����нӴ�ʱ�����γ�ԭ��أ�������ǿ�Ľ������ȱ���ʴ��������һԭ����Ϊ�˱����ִ��ĸ�����ǣ�ͨ������ʻ���ִ����������
��4��Ϊ������ijЩ��˼�����չ����Ҫ�������ֺϳ����������ͺϽ���ϣ�����Ͻ���һ���ܹ���������H2������H2��ϳɽ����⻯��IJ��ϣ��˹��̷�����
��5����������ͭ�������ֽ����Ļ˳���ж����л�ѧ����ʽ��ȷ����
��3Fe+2AlCl3=3FeCl2+2Al ��3Cu+2AlCl3=3CuCl2+2Al ��Fe+CuCl2=FeCl2+Cu
��6��ijʵ���ҷ�Һ�к���HCl��FeCl2��CuCl2�����ʣ��������м����Թ��������ۣ���ַ�Ӧ����ˣ��������к���
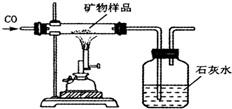
��7��ͬѧ�����������ʵ�鷽���ⶨ�ÿ�����X2O3������������װ�����������ã������е����ʲ��μӷ�Ӧ�����������Ʒ�е�X2O3��ȫ��Ӧ����
��ȡ������Ʒ����������Ʒ��������
�ڲ����Ӧǰ���ƿ��ƿ��������������
�۲����Ӧ����ƿ��ƿ��������������
�ܼ���ó�������Ʒ��x2O3������������
����Ϊ����ʵ�鷽��
���ı�װ�ú�ҩƷ���㻹����ͨ���ⶨ��Щ���ݣ���ͨ������ó�������X2O3������������
�ӻ����Ƕȿ�����װ�õIJ���֮����
��������1�������λ�ڽ���֮���ǽ�������
��2����¯�����з����ķ�Ӧ��һ����̼��ԭ��������
��3������������Ϣ���ԭ��ص�ԭ������ѡ��
��4���ɸ��ݴ���Ͻ�Ĵ���ԭ�����Ͻ����ɽ��з�����
��5�����ݽ����˳������з�����ǰ��Ľ������Ѻ���Ľ�����������Һ���û�������
��6�����ݷ����Ļ�ѧ��Ӧ��������Ϣ�����ۺϷ�����
��7��Ҫ�ⶨ������X2O3���������������Ը������ɵĶ�����̼���������Ƿ�Ӧǰ��������ʵ������������м��㣮
��2����¯�����з����ķ�Ӧ��һ����̼��ԭ��������
��3������������Ϣ���ԭ��ص�ԭ������ѡ��
��4���ɸ��ݴ���Ͻ�Ĵ���ԭ�����Ͻ����ɽ��з�����
��5�����ݽ����˳������з�����ǰ��Ľ������Ѻ���Ľ�����������Һ���û�������
��6�����ݷ����Ļ�ѧ��Ӧ��������Ϣ�����ۺϷ�����
��7��Ҫ�ⶨ������X2O3���������������Ը������ɵĶ�����̼���������Ƿ�Ӧǰ��������ʵ������������м��㣮
����⣺��1�����ݸý��������λ�ڽ���֮�ף�����ȷ���ý���������
�ʴ�Ϊ��B��
��2����¯�����з����ķ�Ӧ��һ����̼��ԭ���������˷�Ӧ�������Ͷ�����̼��
�ʴ�Ϊ��Fe2O3+3CO
2Fe+3CO2
��3������ԭ��ص�ԭ����������ǿ�Ľ������ȱ���ʴ������Ҫ�������壬Ӧѡ�û��õĽ�����п��ͭ���ã�
�ʴ�Ϊ��п��
��4����������֪�ù��������˽����⻯����ǻ�ѧ�仯������Ͻ����ںϽ�һ�����ڻ���
�ʴ�Ϊ����ѧ�������
��5������ͭ�������ֽ����Ļ˳��Ϊ��������ͭ�����ݻ��õĽ������ԴӲ����ý���������Һ���û��������ý������۶��������⣮
�ʴ�Ϊ����
��6���������ۺ���Ȼ�ͭ��Һ��Ӧ���û�������ͭ��������������������������������ͭ��
�ʴ�Ϊ��Fe��Cu
��7��ʯ��ˮ���������Ƶ�ˮ��Һ����������������ˮ�����ʣ��������������٣���һ���ܽ����ɵĶ�����̼ȫ�����գ����Ҵ�װ�ÿ��Կ�����ʯ��ˮֱ��������Ӵ����������տ����еĶ�����̼�����Բ�һ����ȷ���������X2O3���������������������ʵ��ͨ���ⶨ��Ӧǰ��������ʵ���������м��㣬����һ����̼�ж����������β��������
�ʴ�Ϊ����һ���� ���ɵĶ�����̼���岻һ����ʯ��ˮ��ȫ���գ���ʯ��ˮ�������տ����еĶ�����̼�����ֱ�����Ӧǰ�����ܺͿ�����Ʒ������������Ӧ�����ܺ�ʣ�������������������Ӧ��������ʣ����������������β������װ�ã�
�ʴ�Ϊ��B��
��2����¯�����з����ķ�Ӧ��һ����̼��ԭ���������˷�Ӧ�������Ͷ�����̼��
�ʴ�Ϊ��Fe2O3+3CO
| ||
��3������ԭ��ص�ԭ����������ǿ�Ľ������ȱ���ʴ������Ҫ�������壬Ӧѡ�û��õĽ�����п��ͭ���ã�
�ʴ�Ϊ��п��
��4����������֪�ù��������˽����⻯����ǻ�ѧ�仯������Ͻ����ںϽ�һ�����ڻ���
�ʴ�Ϊ����ѧ�������
��5������ͭ�������ֽ����Ļ˳��Ϊ��������ͭ�����ݻ��õĽ������ԴӲ����ý���������Һ���û��������ý������۶��������⣮
�ʴ�Ϊ����
��6���������ۺ���Ȼ�ͭ��Һ��Ӧ���û�������ͭ��������������������������������ͭ��
�ʴ�Ϊ��Fe��Cu
��7��ʯ��ˮ���������Ƶ�ˮ��Һ����������������ˮ�����ʣ��������������٣���һ���ܽ����ɵĶ�����̼ȫ�����գ����Ҵ�װ�ÿ��Կ�����ʯ��ˮֱ��������Ӵ����������տ����еĶ�����̼�����Բ�һ����ȷ���������X2O3���������������������ʵ��ͨ���ⶨ��Ӧǰ��������ʵ���������м��㣬����һ����̼�ж����������β��������
�ʴ�Ϊ����һ���� ���ɵĶ�����̼���岻һ����ʯ��ˮ��ȫ���գ���ʯ��ˮ�������տ����еĶ�����̼�����ֱ�����Ӧǰ�����ܺͿ�����Ʒ������������Ӧ�����ܺ�ʣ�������������������Ӧ��������ʣ����������������β������װ�ã�
�����������漰��֪ʶ��ܶ࣬Ҫ�������Ҫ��ѧ��֪ʶ���֪ʶ�ṹ����������

��ϰ��ϵ�д�
 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д� ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�
�����Ŀ
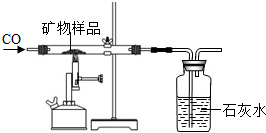 �����ʲ��μӷ�Ӧ�����������Ʒ�е�X2O3��ȫ��Ӧ����
�����ʲ��μӷ�Ӧ�����������Ʒ�е�X2O3��ȫ��Ӧ����