��Ŀ����
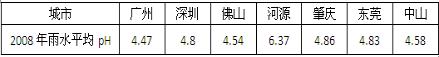
2008����ʡ���ֵ���������ˮƽ��pH���±�����ش��������⣺
| ���� | ���� | ���� | ��ɽ | ��Դ | ���� | ��ݸ | ��ɽ |
| 2008����ˮƽ��pH | 4.47 | 4.8 | 4.54 | 6.37 | 4.86 | 4.83 | 4.58 |
��1���ϱ��оٵ�7�������У�û��������Ⱦ�ĵ�����_______�������������Ҫ������_________�������)�� ��CO ��CO2 ��SO2 ��NO2 ��
��2�����������Ҫ�ɷ�ΪH2SO4��HNO3����д�����л�ѧ����ʽ��
(a)��H2SO4�����긯ʴ�����ҵ�ʯ��ʯ ��
(b)��HNO3��������������������ʯ�ҷ�Ӧ ��
��3��Ϊ�˸������컷��������ӭ������ġ���ɫ���˻ᡱ�����д�ʩ���������������__________�������)�����ƹ������Դ �ڼ�����úֱ����ȼ�� ����̭β������������ ������ȼ���̻����� �����Ʋ��г�ʹ�����ϴ���
��1����3�֣���Դ��1�֣� �ۢܣ�2�֣�
��2����4�֣�H2SO4+CaCO3===CaSO4+CO2��+H2O 2HNO3+Ca(OH)2== Ca(NO3)2+2H2O
��3����1�֣���

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д���7�֣�2008����ʡ���ֵ���������ˮƽ��pH���±�����ش��������⣺
| ���� | ���� | ���� | ��ɽ | ��Դ | ���� | ��ݸ | ��ɽ |
| 2008����ˮƽ��pH | 4.47 | 4.8 | 4.54 | 6.37 | 4.86 | 4.83 | 4.58 |
��2�����������Ҫ�ɷ�ΪH2SO4��HNO3����д�����л�ѧ����ʽ��
(a)��H2SO4�����긯ʴʯ��ʯ ��
(b)��HNO3��������������������ʯ�ҷ�Ӧ ��
��3��Ϊ�˸��ƹ㶫����������ӭ�ӡ���ɫ���˻ᡱ�����д�ʩ���ܼ����������__________�������)�����ƹ������Դ �ڼ�����úֱ����ȼ�� ����̭β������������ ������ȼ���̻����� �����Ʋ��г�ʹ�����ϴ���
| ���� | ���� | ���� | ��ɽ | ��Դ | ���� | ��ݸ | ��ɽ |
| 2008����ˮƽ��pH | 4.47 | 4.8 | 4.54 | 6.37 | 4.86 | 4.83 | 4.58 |
��CO�� ��CO2����SO2����NO2��
��2�����������Ҫ�ɷ�ΪH2SO4��HNO3����д�����л�ѧ����ʽ��
��a����H2SO4�����긯ʴ�����ҵ�ʯ��ʯ ��
��b����HNO3��������������������ʯ�ҷ�Ӧ ��
��3��Ϊ�˸������컷��������ӭ��2010��ġ���ɫ���˻ᡱ�����д�ʩ���ܼ���������� ������ţ������ƹ������Դ���ڼ�����úֱ����ȼ�ϣ�����̭β��������������������ȼ���̻��������Ʋ��г�ʹ�����ϴ���