��Ŀ����
����������ı��⣬���к�ˮռ������ˮ����96.53%���Ժ�ˮ���п�ѧ�Ĵ�������Ϊ�����ṩ��������IJ�Ʒ��
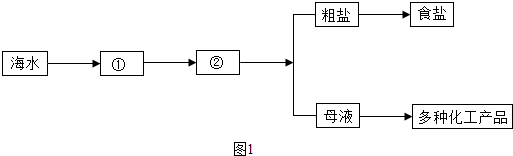
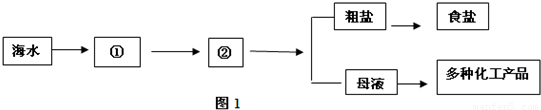
��1����ˮ����
��2������ˮ����������ɲ����������ʵı���ʳ��ˮ��ͨ�������Ƶ��ռ�Ȼ�����Ʒ����ѧ��ӦΪ��2NaCl+2H2O
2NaOH+H2��+Cl2��������Ҫͨ������Ƶ��ռ�800�֣�������Ӧ����ʳ�ζ��ٶ֣���д�������Ľ��ⲽ�裩
��1����ˮ����
�����
�����
��ѡ����������������2������ˮ����������ɲ����������ʵı���ʳ��ˮ��ͨ�������Ƶ��ռ�Ȼ�����Ʒ����ѧ��ӦΪ��2NaCl+2H2O
| ||
��������1�����ݺ�ˮ���ɶ���������ɽ��н��
��2�������ռ���������û�ѧ��Ӧ����ʽ��������μӷ�Ӧ��ʳ�ε��������н��
��2�������ռ���������û�ѧ��Ӧ����ʽ��������μӷ�Ӧ��ʳ�ε��������н��
����⣺��1����ˮ���ɶ���������ɣ����ڻ����������
��2����������Ӧ����ʳ�ε�����Ϊx
2NaCl+2H2O
2NaOH+H2��+Cl2��
117 80
x 800t
=
x=1170t
������Ҫͨ������Ƶ��ռ�800�֣�������Ӧ����ʳ��1170�֣�
��2����������Ӧ����ʳ�ε�����Ϊx
2NaCl+2H2O
| ||
117 80
x 800t
| 117 |
| x |
| 80 |
| 800t |
x=1170t
������Ҫͨ������Ƶ��ռ�800�֣�������Ӧ����ʳ��1170�֣�
���������⿼���˵��ʳ��ˮ��ԭ��Ӧ�ã���ѧ����ʽ�ļ��㷽������Ŀ��

��ϰ��ϵ�д�
 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�
�����Ŀ