��Ŀ����
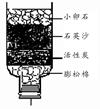
ˮ�����������в���ȱ�ٵ����ʡ�
��1�����о�ˮ�����У�ͨ�����ڳ�ȥˮ�����������ʵ��� ����ˮ�̶���ߵ��� ��
��2������ˮ�����ö�������(ClO2)������������Ԫ�صĻ��ϼ�Ϊ__________��
��3������ˮ�к�������Ca(HCO3)2�ȿ����Ի������ˮʱCa(HCO3)2�����ֽⷴӦ�����������Ե�̼���(CaCO3)��ˮ�Ͷ�����̼������Ǻ��г���ˮ����ԭ��֮һ����д��Ca(HCO3)2���ȷֽ�ķ��ű���ʽ_________��
��1�����о�ˮ�����У�ͨ�����ڳ�ȥˮ�����������ʵ��� ����ˮ�̶���ߵ��� ��
| A������ | B����� | C������ | D������ |
��3������ˮ�к�������Ca(HCO3)2�ȿ����Ի������ˮʱCa(HCO3)2�����ֽⷴӦ�����������Ե�̼���(CaCO3)��ˮ�Ͷ�����̼������Ǻ��г���ˮ����ԭ��֮һ����д��Ca(HCO3)2���ȷֽ�ķ��ű���ʽ_________��
��1��A��C ��2��4+ ��3��Ca��HCO3��2 ���� ��CaCO3 +H2O+CO2��
�����������1�����˿��Ѳ�����ˮ�����ʳ�ȥ��������Եõ�����������ˮ��ͨ�����ڳ�ȥˮ�����������ʵ��ǹ��ˣ���ˮ�̶���ߵ��������A��C����2���ڻ������У�Ԫ�ػ��ϼ۵Ĵ�����Ϊ�㡣�ڶ��������У���Ԫ�صĻ��ϼ���-2�ۣ����������Ԫ�صĻ��ϼ�Ϊ+4�ۡ����+4��

��ϰ��ϵ�д�
�����Ŀ


 ��ͬѧ��������ʳ������ѡ����Ϊ�����ṩ�϶�ά���ص�һ��ʳ���� ����д��ѡʳ������ƣ���
��ͬѧ��������ʳ������ѡ����Ϊ�����ṩ�϶�ά���ص�һ��ʳ���� ����д��ѡʳ������ƣ���