��Ŀ����
��H��C��O��Na��Cl����Ԫ���е�һ�ֻ������ɰ������г��л�ѧ�������ʣ�����������������������������A�����������Ҫ�����壬��һ�������¿���ת����B��
��B����C��Ӧ����D��E������B��E������������
��D����B��E�������Ϸ�Ӧ����F��
��D��F������G��Ӧ����H��ÿ������������H����ά��������ˮ�ֺ���Һ�㶨��pH����Ҫ���á�
����������ش��������⣺
��1��A���ʵĻ�ѧʽΪ ��C�������ճ������е���ҪӦ���� ����һ������
��2��D��F�Ļ�ѧ����ʽΪ ��
��3��F��G��Ӧ�Ļ�ѧ����ʽΪ ��
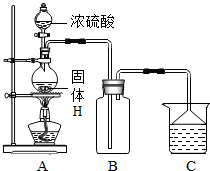
��4��ʵ������HΪԭ�ϣ�������ͼ��ʾװ����ȡG��A��������Ӧ�Ļ�ѧ����ʽΪ �����ڸ�ʵ�飬����˵������ȷ���ǣ�����ĸ�� ��
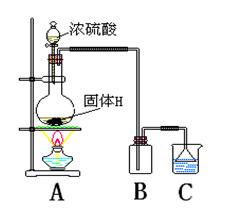
A���Ʊ�װ����ʵ������ȡCO2��ͬ
B����ʵ���β�������ü�Һ����
C����������ռ��������������ռ�������ȫ��ͬ
D������������Ƿ��ռ�������������ƿ�еμ���ɫʯ����Һ
��1��O2�� �������
��2��Na2CO3 + H2O + CO2 = 2NaHCO3
��3��NaHCO3 + HCl =" NaCl" + H2O + CO2��
��4��2NaCl + H2SO4 Na2SO4 + 2HCl�� ��ACD����:
Na2SO4 + 2HCl�� ��ACD����:
��
��2��Na2CO3 + H2O + CO2 = 2NaHCO3
��3��NaHCO3 + HCl =" NaCl" + H2O + CO2��
��4��2NaCl + H2SO4
 Na2SO4 + 2HCl�� ��ACD����:
Na2SO4 + 2HCl�� ��ACD����:��

��ϰ��ϵ�д�
 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
�����Ŀ


 ��H��C��O��Na��Cl����Ԫ���е�һ�ֻ������ɰ������г��л�ѧ�������ʣ���������������������������
��H��C��O��Na��Cl����Ԫ���е�һ�ֻ������ɰ������г��л�ѧ�������ʣ��������������������������� ��������ĸ��ʾ���dz��л�ѧ���������ʣ�������H��C��O��Na��S��Ca��Fe�еļ���Ԫ����ɣ�
��������ĸ��ʾ���dz��л�ѧ���������ʣ�������H��C��O��Na��S��Ca��Fe�еļ���Ԫ����ɣ�