��Ŀ����
�����ѧϰ��������ἴ�̲ġ�����ѧ����ʳס�С�������Դ�����Ͽ�ѧ��ҽ�������ȷ���Խ��Խ��������Լ��ļ�ֵ��
��1��֥�齴���зḻ��Ӫ����ͼ1ΪijƷ��֥�齴�̱��һ���֣����иơ���������ָ ������ĸ��ţ���
A������ B��ԭ�� C������ D��Ԫ��
��2��Ϊ��ֹ������ʴ����������Ʒ����ˢ�����۵ķ����ᣬ������Ϊ���ڿ����������ұ����������γ�һ�����ܵ�����Ĥ����ѧ����ʽ�� ��
��3���ϵ����Ǧ���ӡ������ؽ�����������ˮԴ����Ⱦ�dz����أ�С���ӷϸɵ���л����й����ʲ�����̽����
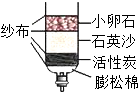
������ͼ2����и����ʽ��з��࣬���пɻ��յĽ��������� ����дһ�����ɣ�
����ȡ�������̣���ȥ���������е�����̼�ۣ�����̼����������̲���Ӧ�����ɲ��õķ����� ��
��4�����������䡱�����ѽ���Ȼ�����뵤���������йܵ�ú���û���½����������Ȼ����
��ʵ��֤������ͬ��ͬѹ�£���ͬ������κ������к�����ͬ�ķ�����������ͬ��ͬѹ�£���ͬ�����CO��CH4�ֱ��ڳ��ȼ�գ����������϶���� ��
��ȼ���������塢��ȼ���ܡ��������ܡ����ӿ��صȲ�����ɣ�Ŀǰ�ܵ�ú���û�������Ȼ���������ȼ������Ը��죮�����ȼ���ܵ�ֱ�����䣬��������ܵ�ֱ����ԭ�����Ӧ ����������١���
��1.6g������������һ�������·�Ӧ������ȫȼ�գ���������CO��CO2��H2O��������Ϊ7.6g����Ҫʹ�������ļ�����ȫȼ�գ����貹�� g������
���𰸡���������1������Ʒ��֥�齴�иơ��������Ĵ�����������
��2����������������Ӧ�������ܵ���������������
��3������ͼ2����и������������ܻ��յĽ������ϣ����������ʵ��������������ӣ�
��4��������ͬ�����CO��CH4�ֱ��ڳ��ȼ�յķ�Ӧ��������Ȼ�����ü�����ȫȼ�����ɶ�����̼��ˮ���������������貹���������
����⣺��1����Ʒ��֥�齴�иơ��������Ĵ����ڻ������У�������ĸơ�������ָԪ�أ��ʴ�Ϊ��D��
��2�����ڿ����������ұ����������γ�һ�����ܵ�����Ĥ����ӦΪ4Al+3O2�T2Al2O3���ʴ�Ϊ��4Al+3O2�T2Al2O3��
��3������ͼ��֪������е�ͭñ��пƤ���ǽ������ϣ�����Ի������ã��ʴ�Ϊ��ͭ��п��
����������̲�ȼ�գ���̼����ȼ���������壬���ڿ��������տɶ��������е�����̼�ۣ��ʴ�Ϊ���ڿ��������գ�
��4������CO+ O2
O2 CO2��CH4+2O2
CO2��CH4+2O2 CO2+H2O����Ȼ��ͬ�����CO��CH4�ֱ���ȼ�ռ������ĵ������࣬�ʴ�Ϊ�����飻
CO2+H2O����Ȼ��ͬ�����CO��CH4�ֱ���ȼ�ռ������ĵ������࣬�ʴ�Ϊ�����飻
�ڹܵ�ú���û�������Ȼ��ʱ������Ȼ������Ҫ�ɷ�Ϊ���飬��ͬ�����CO��CH4�ֱ���ȼ�ռ������ĵ������࣬��Ӧ����������ܵ�ֱ�����ʴ�Ϊ������
��1.6g������������ȫ��Ӧ���ɵĶ�����̼������Ϊ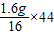 =4.4g�����ɵ�ˮ������Ϊ
=4.4g�����ɵ�ˮ������Ϊ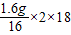 =3.6g������ȫȼ���������������Ϊ4.4g+3.6g=8g���ֲ���ȫȼ��������CO��CO2��H2O��������Ϊ7.6g������Ҫ������������Ϊ8g-7.6g=0.4g���ʴ�Ϊ��0.4��
=3.6g������ȫȼ���������������Ϊ4.4g+3.6g=8g���ֲ���ȫȼ��������CO��CO2��H2O��������Ϊ7.6g������Ҫ������������Ϊ8g-7.6g=0.4g���ʴ�Ϊ��0.4��
���������⿼��֪ʶ��࣬ע�ضԻ���֪ʶ�Ŀ��飬��Ϣ��ǿ���Ϻõ�ѵ��ѧ��������Ϣ����ѧ֪ʶ�Ľ������������������
��2����������������Ӧ�������ܵ���������������
��3������ͼ2����и������������ܻ��յĽ������ϣ����������ʵ��������������ӣ�
��4��������ͬ�����CO��CH4�ֱ��ڳ��ȼ�յķ�Ӧ��������Ȼ�����ü�����ȫȼ�����ɶ�����̼��ˮ���������������貹���������
����⣺��1����Ʒ��֥�齴�иơ��������Ĵ����ڻ������У�������ĸơ�������ָԪ�أ��ʴ�Ϊ��D��
��2�����ڿ����������ұ����������γ�һ�����ܵ�����Ĥ����ӦΪ4Al+3O2�T2Al2O3���ʴ�Ϊ��4Al+3O2�T2Al2O3��
��3������ͼ��֪������е�ͭñ��пƤ���ǽ������ϣ�����Ի������ã��ʴ�Ϊ��ͭ��п��
����������̲�ȼ�գ���̼����ȼ���������壬���ڿ��������տɶ��������е�����̼�ۣ��ʴ�Ϊ���ڿ��������գ�
��4������CO+
 O2
O2 CO2��CH4+2O2
CO2��CH4+2O2 CO2+H2O����Ȼ��ͬ�����CO��CH4�ֱ���ȼ�ռ������ĵ������࣬�ʴ�Ϊ�����飻
CO2+H2O����Ȼ��ͬ�����CO��CH4�ֱ���ȼ�ռ������ĵ������࣬�ʴ�Ϊ�����飻 �ڹܵ�ú���û�������Ȼ��ʱ������Ȼ������Ҫ�ɷ�Ϊ���飬��ͬ�����CO��CH4�ֱ���ȼ�ռ������ĵ������࣬��Ӧ����������ܵ�ֱ�����ʴ�Ϊ������
��1.6g������������ȫ��Ӧ���ɵĶ�����̼������Ϊ
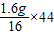 =4.4g�����ɵ�ˮ������Ϊ
=4.4g�����ɵ�ˮ������Ϊ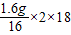 =3.6g������ȫȼ���������������Ϊ4.4g+3.6g=8g���ֲ���ȫȼ��������CO��CO2��H2O��������Ϊ7.6g������Ҫ������������Ϊ8g-7.6g=0.4g���ʴ�Ϊ��0.4��
=3.6g������ȫȼ���������������Ϊ4.4g+3.6g=8g���ֲ���ȫȼ��������CO��CO2��H2O��������Ϊ7.6g������Ҫ������������Ϊ8g-7.6g=0.4g���ʴ�Ϊ��0.4�����������⿼��֪ʶ��࣬ע�ضԻ���֪ʶ�Ŀ��飬��Ϣ��ǿ���Ϻõ�ѵ��ѧ��������Ϣ����ѧ֪ʶ�Ľ������������������

��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ


 �����ѧϰ��������ἴ�̲ġ�����ѧ����ʳס�С�������Դ�����Ͽ�ѧ��ҽ�������ȷ���Խ��Խ��������Լ��ļ�ֵ��
�����ѧϰ��������ἴ�̲ġ�����ѧ����ʳס�С�������Դ�����Ͽ�ѧ��ҽ�������ȷ���Խ��Խ��������Լ��ļ�ֵ��
