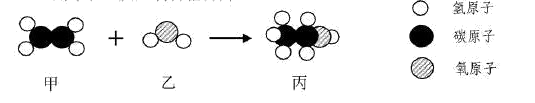��Ŀ����
��5�֣���ѧ�����������ܲ��ɷ֡�
��1������ͨ��ʳ���ȡ����Ӫ����
��ˮ�����߲˸�����Ӫ������Ҫ��ˮ�� ��
��Ϊ�˷�ֹ�������ɣ�����ÿ����������㹻���� Ԫ�ء�
��2�����г�����Ʒ��ʹ�õ���Ҫ���ϣ������л��ϳɲ��ϵ��� ������ĸ��ţ���
��3�������Ի�ʯȼ��Ϊ��Ҫ��Դ��
����Ȼ��ȼ�յĻ�ѧ����ʽΪ ��
���������������ڼ��ٿ�����Ⱦ���� ������ĸ��ţ���
| A���������̫���� | B��������չ�������� |
| C������ʹ�û�ʯȼ�� | D��Ϊ������У�����ʹ��˽�ҳ� |
��1����ά���� �ڸ� ��2��C��D ��3��CH4 + 2O2 ��ȼ CO2 +2 H2O ��AC
����

�����й���Դ����Դ��������ȷ���ǡ���������������������������������( )
| A��Ŀǰ�������������࣬ʹ����㷺�Ľ������� |
| B��ʯ��ͨ���ֽⷴӦ���Եõ����͡�ú�͡�����ú�͵� |
| C�������к��еĻ�ѧԪ����80���֣����к�������Ԫ������Ԫ�� |
| D�������еĸ��ֳɷֿ��Թ㷺�����������ʡ����֡����䡢���Դ������ |
��10�֣���ѧ������������ϢϢ��ء�������ѧ��֪ʶ�ش��������⣺
��1���˺������ò�����CO2�����ܼ�ʱ�ų���������ѪҺ�л�ʹѪҺ��pH ��ѡ����䡱�������С������
��2��������β���ŷſڼ�װ������������������β���е��к�����CO��NO�������·�Ӧ��
2CO + 2NO ���� N2 + 2X ����X�Ļ�ѧʽΪ ��
��3����Ȼ������õĻ�ʯȼ��֮һ����Ҫ�ɷ��Ǽ��飨CH4������ȼ�յĻ�ѧ����ʽ
�� ��
��4��������Ϊ��ȫ����ȡ���� ������ţ���
| A��С��ʹ��Ũ��Ũ�� | B��ʹ��ȼ����ˮ����ԡʱ�������� |
| C��ù��Ĵ���ϴ�������ʳ�� | D�������δ�����IJ˽�ǰ���ƻ����� |
2011���绯ѧ��������ǡ��������������δ����������������������һ�������( )
| A���ϳ�ҩ������������ |
| B��������չ�������磬���տ�������������� |
| C��Ϊ���ũ����IJ�����Ӧ����ʹ�û��ʺ�ũҩ |
| D��Ϊ����ʳ���ɫ���㡢ζ����ֹ���ʣ��������м��������ʳƷ���Ӽ� |