��Ŀ����
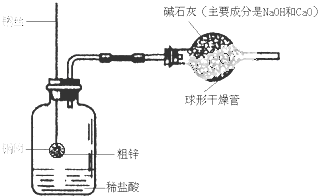 ijͬѧ�����һ������ͼ��ʾ��װ�ã����ø�װ�òⶨ��п��Ʒ�ĺ�п����
ijͬѧ�����һ������ͼ��ʾ��װ�ã����ø�װ�òⶨ��п��Ʒ�ĺ�п����
��1������10.0g��п����ͭ���У���ͼ��������װ���ԺƵ�������ҩƷ������Ϊ120.0g��
��2����ͭ����������ϡ�����У���ַ�Ӧ�����Թ۲쵽��ʵ������Ϊ________����Ӧ�Ļ�ѧ����ʽΪ________��
��3����Ӧ��ȫ�Ƶ�װ��������Ϊ119.8g�����п�Ĵ���Ϊ________��
��4����ʵ���м�ʯ�ҵ������Ǹ������ͬʱ��ֹϡ����ӷ������Ȼ���������ɢ�������У������ü�ʯ�ң������ⶨ�Ĵ�п����________���ƫ����ƫС������Ӱ�족��
��5��������п����ʯ��ʯ��ԭʵ�鷽��________����ܡ����ܡ�������ʯ��ʯ��Ʒ���ȵIJⶨ��������________��
�⣺��2��п�����ᷴӦ�����Ȼ�п���������������ݣ���Ӧ�Ļ�ѧ����ʽΪ Zn+2HCl=ZnCl2+H2����
��3����μӷ�Ӧп������Ϊx������������=120g-119.8g=0.2g��
Zn+2HCl=ZnCl2+H2��
65 2
x 0.2g

x=0.2g
��п�Ĵ���=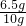 ��100%=65%
��100%=65%
�𣺴�п�Ĵ���Ϊ65%��
��4��ϡ����ӷ������Ȼ���������ɢ�������У�ʹװ����������С������������ƫ�����п������Ҳƫ�����ⶨ�Ĵ�п����ƫ��
��5����ʯ�������ն�����̼��ˮ���������壬���²��ܲ��CO2��������
�ʴ�Ϊ����2���������ݣ�Zn+2HCl=ZnCl2+H2����
��3��65%��
��4��ƫ��
��5�����ܣ� ��ʯ�������ն�����̼��ˮ���������壬���²��ܲ��CO2��������
��������2������п�����ᷴӦ�����Ȼ�п���������������ݽ��н��
��3����������������=120g-119.8g=0.2g�����ݻ�ѧ����ʽ�б���ʽ���п���������н��
��4������ϡ����ӷ������Ȼ���������ɢ�������У�ʹװ����������С������������ƫ�����п������Ҳƫ�����ⶨ�Ĵ�п����ƫ����н��
��5�����ݼ�ʯ�������ն�����̼��ˮ���������壬���²��ܲ��CO2���������н��
���������⿼����ͼʾ�ͻ�ѧ��Ӧ���ϵ�ͨ����ѧ����ʽ���м��㣬�����Ѷ��Ӵ����Ĺؼ����ܹ����������ҳ�������ϵ�����շ�Ӧ���������ѧ�����������Ҫ��ϸߣ�
��3����μӷ�Ӧп������Ϊx������������=120g-119.8g=0.2g��
Zn+2HCl=ZnCl2+H2��
65 2
x 0.2g

x=0.2g
��п�Ĵ���=
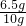 ��100%=65%
��100%=65%�𣺴�п�Ĵ���Ϊ65%��
��4��ϡ����ӷ������Ȼ���������ɢ�������У�ʹװ����������С������������ƫ�����п������Ҳƫ�����ⶨ�Ĵ�п����ƫ��
��5����ʯ�������ն�����̼��ˮ���������壬���²��ܲ��CO2��������
�ʴ�Ϊ����2���������ݣ�Zn+2HCl=ZnCl2+H2����
��3��65%��
��4��ƫ��
��5�����ܣ� ��ʯ�������ն�����̼��ˮ���������壬���²��ܲ��CO2��������
��������2������п�����ᷴӦ�����Ȼ�п���������������ݽ��н��
��3����������������=120g-119.8g=0.2g�����ݻ�ѧ����ʽ�б���ʽ���п���������н��
��4������ϡ����ӷ������Ȼ���������ɢ�������У�ʹװ����������С������������ƫ�����п������Ҳƫ�����ⶨ�Ĵ�п����ƫ����н��
��5�����ݼ�ʯ�������ն�����̼��ˮ���������壬���²��ܲ��CO2���������н��
���������⿼����ͼʾ�ͻ�ѧ��Ӧ���ϵ�ͨ����ѧ����ʽ���м��㣬�����Ѷ��Ӵ����Ĺؼ����ܹ����������ҳ�������ϵ�����շ�Ӧ���������ѧ�����������Ҫ��ϸߣ�

��ϰ��ϵ�д�
 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
�����Ŀ




