��Ŀ����
����Ŀ��ȼ�յ�֪ʶ�����Ա��������Ʋ���ȫ�dz���Ҫ��Ҳ�Ի�������������Ҫ��ֵ�� 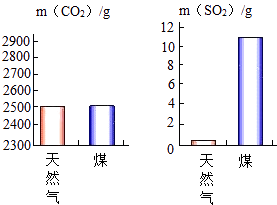
��1����ȥ������ij�տ���һ�˿Ͳ��ұ��ƾ��������ˣ���ʱ�ڳ������õ����оȻ�ʽ������Ч����
A�����·��Ĵ�Ȼ� B����ˮ C���øɷ������
�ڸ��Ż�Ĺ˿����Ż��Ҳ��Ӧ�õ������ܣ�Ӧ�þ͵ط�����𣬴�����ԭ���Դ˵Ľ����ǣ� �� ����㵱ʱҲ�ڳ�������Ϊ�����Բ���ʲô��ʩ��Ѹ�����ȱ������˵Ĺ˿ͣ� ��
��2����д���ƾ�ȼ�յĻ�ѧ����ʽ�� ��
��3�����ȼ��1000g��Ȼ����ú��������CO2��SO2�����������ͼ��ʾ������ͼʾ����������˵����ȷ�������������
A.úȼ�ղ������������������
B.����Ȼ���в�����Ԫ��
C.úȼ�նԻ���Ӱ���С
D.ú����Ȼ����ȼ�ն��������������
��4�����ǿ��Բ�ȡ��Щ��ʩ������úȼ�ղ�����SO2�Դ�������Ⱦ ��
���𰸡�
��1��C����������������ˮ��ϴ���˲�λ
��2��C2H5OH+3O2 ![]() 2CO2+3H2O
2CO2+3H2O
��3��AD
��4���Ľ�ȼ��װ�ú�ȼ�ռ�������չ����Դ
���������⣺��1���ƾ�ȼ����ɵĻ��֣��������·��Ĵ�Ҳ������ˮ����𣬿��ܻᵼ�»������ӣ����øɷ����������𣻹��C���͵ط�������Ǹ���������𣬱������˵IJ���Ƥ��������ˮ����ϴ��������ֹ��һ���������������������ˮ��ϴ���˲�λ����2���ƾ�ȼ�յĻ�ѧ����ʽΪ��C2H5OH+3O2 ![]() 2CO2+3H2O�����C2H5OH+3O2
2CO2+3H2O�����C2H5OH+3O2 ![]() 2CO2+3H2O����3��A������ͼʾ��֪��úȼ�ղ����Ķ�������Ķ࣬���������꣬��A��ȷ��B����Ȼ��ȼ�����ɶ����������Ը���Ȼ���к���Ԫ�أ���B����C������ͼʾ��֪����Ȼ����úȼ�պ����������̼���壬����������Ȼ����ȫȼ�ղ����Ķ�������ȵ�������ú��ȫȼ�ղ����Ķ��������ٺܶ࣬���ȼú�����������꣬�Ի���Ӱ��Ƚϴ�C����D��ú����Ȼ����ȼ�ն����ɶ�����̼�����Զ������������������D��ȷ�����AD����4�����ٶ����������Ⱦ;���У�����ȼú����������ȼúװ�á��Ľ�ȼ��װ�ú�ȼ�ռ������Ľ������豸����չ�ྻú��������չ����Դ�ȣ����Դ��ǣ��Ľ�ȼ��װ�ú�ȼ�ռ�������չ����Դ��
2CO2+3H2O����3��A������ͼʾ��֪��úȼ�ղ����Ķ�������Ķ࣬���������꣬��A��ȷ��B����Ȼ��ȼ�����ɶ����������Ը���Ȼ���к���Ԫ�أ���B����C������ͼʾ��֪����Ȼ����úȼ�պ����������̼���壬����������Ȼ����ȫȼ�ղ����Ķ�������ȵ�������ú��ȫȼ�ղ����Ķ��������ٺܶ࣬���ȼú�����������꣬�Ի���Ӱ��Ƚϴ�C����D��ú����Ȼ����ȼ�ն����ɶ�����̼�����Զ������������������D��ȷ�����AD����4�����ٶ����������Ⱦ;���У�����ȼú����������ȼúװ�á��Ľ�ȼ��װ�ú�ȼ�ռ������Ľ������豸����չ�ྻú��������չ����Դ�ȣ����Դ��ǣ��Ľ�ȼ��װ�ú�ȼ�ռ�������չ����Դ��
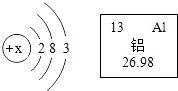
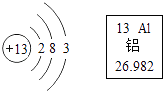
�����㾫����������д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ�ͳ���ȼ�ϵ�ʹ������Ի�����Ӱ���ǽ����ĸ�������Ҫ֪��ע�⣺a����ƽ b������ c�����ţ�úȼ���ŷŵ���Ⱦ�SO2��NO2���������꣩��CO���̳��ȣ�ʯ��ȼ������β������Ⱦ�CO��δȼ�յ�̼�⻯��������������Ǧ��������̳�����Ȼ���ǽ�������Դ��

 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�