��Ŀ����
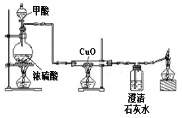
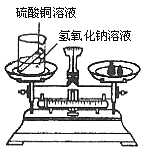
����Ŀ����7�֣�ij��ȤС��Ϊ����֤�����غ㶨�ɣ����ձ���ʢ��20g������������Ϊ20%������������Һ�Թ���װ��20g����ͭ��Һ����ͼ��ʾ��������ƽ�ϳ�������ʱ��ƽƽ�⡣Ȼ���Թ���ҩƷ�����ձ��ڣ��Թܲ�ȡ������ǡ����ȫ��Ӧ��
�Ը���Ҫ�ش��������⣺
��1��д��ʵ������й۲쵽��һ��ʵ������ ________________________________________________��
��2����Ӧ��������ƽָ���__________ ���ƫ��ƫ�ҡ���������ƫת��֮һ����
��3���Լ���ǡ����ȫ��Ӧʱ���ò�������Һ������������ȷ��0��1g��
���𰸡���1����Һ����ɫ��Ϊ��ɫ��������ɫ������
��2��������ƫת��
��3���⣺������������ͭ������Ϊy��
��������֪���������Ƶ�����=20g*20%=4g
CuSO4+2NaOH==Cu(OH)2��+Na2SO4
80 98
4g y
80/98=4g/y y=4��9g
��Ӧ��������������Һ������=20g+20g-4��9g=35��1g
��ǡ����ȫ��Ӧʱ���ò�������Һ������Ϊ35��1g��
��������
�����������1������ͭ���������Ʒ�Ӧ��������ɫ����������ͭ����2�����������غ㶨�ɿ�֪����Ӧǰ�����ʵ�������û�з����仯��������ƽָ�벻�ᷢ��ƫת����3����������������Һ�����ʵ����������û�ѧ����ʽ��������������ͭ���������������������غ㶨�ɼ�����������Һ����������Ӧǰ��Һ����������ȥ���ɳ�����������

����Ŀ����������ʵ���Ͳ���ȷ����
ѡ�� | �� ʵ | �� �� |
A | ���¼��е�ˮ���������������� | ԭ�ӵ������������ |
B | һ��ˮ�д�Լ��1��67��1021��ˮ���� | ���Ӻ�С |
C | ���������еľƾ����� | �����ڲ����˶� |
D | ���ȵ��������г���̥���ױ��� | �����¶ȸߣ����Ӽ������ |