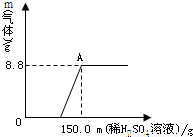
��Ŀ����
��2010?��̶��2009��12���ڸ籾�����ٿ������Ϲ�����仯��ᣬ����̼�����Ϊ���Ż��⣮����̼�����ָ������Ϣʱ�����������٣��Ӷ�����̼�ر��Ƕ�����̼���ŷţ���1�����������в����ϡ���̼���������ǣ�����ţ���
�ٶ��õ��ʡ�QQ�ȼ�ʱͨѶ���ߣ����ô����ӡ��
�������г��������°�
�۴���ʹ�û�ʯȼ��
���Ż�������ƣ���ǿ������Ȼ�ɹ⣬���������õ�
��2����������һ�����ճ������з��ϡ���̼�������������
��3����ѧ�������о���������̼��������Ӧ���ɼ��顢���ᣨHCOOH���Ȼ���ԭ�ϣ����顢�������ڣ��������л������
���𰸡���������1�����ݵ�̼������з�������̼������Ǽ��ٶ�����̼���ŷţ�
��2�����ݡ���̼����ĺ���ش𣬵�Ҫע��ʵ��Ҫ�ճ������У�
��3�������л�������Ķ���ش�
����⣺��1�������г��������°��ܼ��ٽ�ͨ���ߵ�ʹ�ã��Ӷ����ٶ�����̼���ŷţ����õ��ʡ�QQ�ȼ�ʱͨѶ���ߣ����ô����ӡ���ܼ��ٶ�����̼���ŷţ��Ż�������ƣ���ǿ������Ȼ�ɹ⣬���������õ磬Ҳ���Լ��ٷ�������ȼ�ϣ�ͬ�����ٶ�����̼���ŷţ�ֻ�л�ʯȼ�ϵĴ���ʹ�û����Ӷ�����̼���ŷţ���ѡ�ۣ�
�ʴ�Ϊ���ۣ�
��2��Ҫ���ٴ����еĶ�����̼��һ����������ŷ���������Ҫͨ������������ղ��ֶ�����̼���������ճ������з��ϡ���̼����������ܶ࣬������ᳫʹ��̫������ˮ����ֽ˫���ã����д���������������ֹصƵȣ�
�ʴ�Ϊ�������ᳫʹ��̫������ˮ����
��3���л���������ָ��̼�Ļ����̼�������̼�ᡢ̼���γ��⣩�����顢���ᣨHCOOH���ȷ����л�������Ķ��壮
�ʴ�Ϊ���л��
������������Ҫ����ѧ��������ѧ��ѧ֪ʶ�ۺϷ����ͽ��ʵ�������������֪ʶ���漰�Ϲ㣬˼ά��ȴ�ǿ����ѧ������֪ʶ��������
��2�����ݡ���̼����ĺ���ش𣬵�Ҫע��ʵ��Ҫ�ճ������У�
��3�������л�������Ķ���ش�
����⣺��1�������г��������°��ܼ��ٽ�ͨ���ߵ�ʹ�ã��Ӷ����ٶ�����̼���ŷţ����õ��ʡ�QQ�ȼ�ʱͨѶ���ߣ����ô����ӡ���ܼ��ٶ�����̼���ŷţ��Ż�������ƣ���ǿ������Ȼ�ɹ⣬���������õ磬Ҳ���Լ��ٷ�������ȼ�ϣ�ͬ�����ٶ�����̼���ŷţ�ֻ�л�ʯȼ�ϵĴ���ʹ�û����Ӷ�����̼���ŷţ���ѡ�ۣ�
�ʴ�Ϊ���ۣ�
��2��Ҫ���ٴ����еĶ�����̼��һ����������ŷ���������Ҫͨ������������ղ��ֶ�����̼���������ճ������з��ϡ���̼����������ܶ࣬������ᳫʹ��̫������ˮ����ֽ˫���ã����д���������������ֹصƵȣ�
�ʴ�Ϊ�������ᳫʹ��̫������ˮ����
��3���л���������ָ��̼�Ļ����̼�������̼�ᡢ̼���γ��⣩�����顢���ᣨHCOOH���ȷ����л�������Ķ��壮
�ʴ�Ϊ���л��
������������Ҫ����ѧ��������ѧ��ѧ֪ʶ�ۺϷ����ͽ��ʵ�������������֪ʶ���漰�Ϲ㣬˼ά��ȴ�ǿ����ѧ������֪ʶ��������

��ϰ��ϵ�д�
�����Ŀ
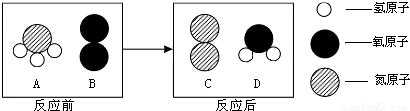
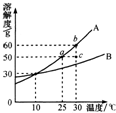 ��2010?��̶��ͼ��A��B���ֹ������ʵ��ܽ�����ߣ���ͼ�ش�
��2010?��̶��ͼ��A��B���ֹ������ʵ��ܽ�����ߣ���ͼ�ش�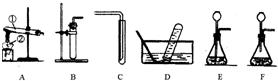 ��2010?��̶����ͼ��A��B��ʵ������ȡ����ʱ�ij���װ�ã��밴Ҫ����գ�
��2010?��̶����ͼ��A��B��ʵ������ȡ����ʱ�ij���װ�ã��밴Ҫ����գ�