��Ŀ����
�������£�ʵ�����Ⱥ����ѧ���ͬѧһ�����ŵ綯���г����̤��ɷ磬
��1�����е綯���г���������ʹ�õIJ����У������л��ϳɲ��ϵ���______
A������ֳ�Ȧ������B�����ϳ��𡡡��� C���Ͻ��塡���� D���������⾵
��2��ͬѧ��Я����ʳƷ�У����������ʵ���______
A�������ײˡ����� B������������� C����������������D��ƻ��
��3���������ʵ�鷽������ͬѧ��ȷ��ʳƷ��װ���Ǿ���ϩ���ϻ��Ǿ�����ϩ���ϣ�д�����������ۣ���______
��4��ͬѧ�ǿ���ũ����ׯ�ڵ�Ҷɫ���ƣ�˵��ֲ��ȱ______�ʣ���ʩ���Ȼ�泥�����������ʯ�һ��ʹ�ã�ԭ���ǣ�______�����û�ѧ����ʽ��ʾ��
��5��̤��ʱ��ͬѧ��С�ı��ó涣ҧ���ó��ܷ��ڳ����ᣩ���������ʿ�����ͿĨ�Լ�����ʹ����______
A��ʳ�ס�������B��ʳ��ˮ��������C������ˮ��������D���ռ���Һ��
��6��ͬѧ�Ǻ��������µĿ�������̾����Ȼ�������Ȼ�������Ķ�����̼����Ҫ;�����û�ѧ����ʽ��ʾ��______
��7��ͬѧ��Ұ�����������������ʣ����пɱ����յ���______
A����ֽ������B����ʣ��ƻ�������� C�������ޡ�������D�������ǣ�
�⣺��1���л��ϳɲ�����Ҫ�����ϳ������Ϻͺϳ���ά�������ϳ��������л��ϳɲ��ϣ�������ֳ�Ȧ���Ͻ������������ϣ��������⾵�����ǽ������ϣ�
��2������ʳ�������е�Ӫ�����ʿ�֪���ײ˺�ƻ����Ҫ����ά���ء������Ҫ���е��۵ȡ����������������ʣ�
��3����������ȼ��ʱ���������ʵ�鼴�ɣ����û��յķ�����������ϩȼ��ʱ����𣬶�����Ũ�̣��ʹ̱����壬����ϩ����Ϩ�𣬶��Ǽ���ȼ�գ����̻������̣�������ʯ��ζ��
��4��ׯ��ȱ�ٵ���ʱҶƬ�ᷢ�ƣ����Կ���ʹ�õ��ʽ��л��⣻�̬���ʲ�����������ʻ��ʹ�ûᵼ�µ��ʵ�ʧЧ���䷽��ʽΪ��2NH4Cl+Ca��OH��2�TCaCl2+2NH3��+2H2O��
��5��������������ԣ����Լ�����ʹ��Ҫ���ĵ����ᣬѡ���д��ᡢ�Ȼ��ƾ�������֮��Ӧ�����Լ��Եķ���ˮ���ռ���Һ�����������ᣬ���ռ���Һ���и�ʴ�ԣ�����һ����÷���ˮ���������⣻
��6����Ȼ���й�����������Ĵ����Ķ�����̼���䷴Ӧ�ı���ʽΪ��6CO2+6H2O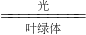 C6H12O6+6O2��
C6H12O6+6O2��
��7����������������Դ����������ѡ���е�������֪��ֽ���������ɻ��գ�����ʣ��ƻ�������������ڲ��ɻ��յ�������
�ʴ�Ϊ����1��B����2��C����3�����û��յķ�����ȼ��ʱ����𣬶�����Ũ�̣��ʹ̱������Ǿ�����ϩ������Ϩ�����ʱ����ȼ�գ����̻������̣�������ʯ��ζ�Ǿ���ϩ��
��4������2NH4Cl+Ca��OH��2�TCaCl2+2NH3��+2H2O����5��C����6��6CO2+6H2O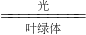 C6H12O6+6O2����7��AC��
C6H12O6+6O2����7��AC��
��������1�������л��ϳɲ��ϵ��йط�Χ�������
��2������ʳ�������е�Ӫ�����ʷ����ɣ�
��3����������ȼ��ʱ������ͬ�������
��4������ׯ��ȱ�ٵ����ǵ�����Լ��̬���ʲ�����������ʻ��ʹ�õ��й�֪ʶ��ɣ�
��5��������������Խ������к���ʵ������������
��6�����ݹ�����������Ĵ����Ķ�����̼��֪ʶ��д��ѧ����ʽ���ɣ�
��7���������������й���������жϣ�
���������⿼���˽�����Ӧ���Լ���������ص��й�֪ʶ����ɴ��⣬�����������еĿα�֪ʶ���У�Ҫ��ͬѧ�Ǽ�ǿ�Ի���֪ʶ�Ĵ������Ա����Ӧ�ã�
��2������ʳ�������е�Ӫ�����ʿ�֪���ײ˺�ƻ����Ҫ����ά���ء������Ҫ���е��۵ȡ����������������ʣ�
��3����������ȼ��ʱ���������ʵ�鼴�ɣ����û��յķ�����������ϩȼ��ʱ����𣬶�����Ũ�̣��ʹ̱����壬����ϩ����Ϩ�𣬶��Ǽ���ȼ�գ����̻������̣�������ʯ��ζ��
��4��ׯ��ȱ�ٵ���ʱҶƬ�ᷢ�ƣ����Կ���ʹ�õ��ʽ��л��⣻�̬���ʲ�����������ʻ��ʹ�ûᵼ�µ��ʵ�ʧЧ���䷽��ʽΪ��2NH4Cl+Ca��OH��2�TCaCl2+2NH3��+2H2O��
��5��������������ԣ����Լ�����ʹ��Ҫ���ĵ����ᣬѡ���д��ᡢ�Ȼ��ƾ�������֮��Ӧ�����Լ��Եķ���ˮ���ռ���Һ�����������ᣬ���ռ���Һ���и�ʴ�ԣ�����һ����÷���ˮ���������⣻
��6����Ȼ���й�����������Ĵ����Ķ�����̼���䷴Ӧ�ı���ʽΪ��6CO2+6H2O
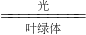 C6H12O6+6O2��
C6H12O6+6O2����7����������������Դ����������ѡ���е�������֪��ֽ���������ɻ��գ�����ʣ��ƻ�������������ڲ��ɻ��յ�������
�ʴ�Ϊ����1��B����2��C����3�����û��յķ�����ȼ��ʱ����𣬶�����Ũ�̣��ʹ̱������Ǿ�����ϩ������Ϩ�����ʱ����ȼ�գ����̻������̣�������ʯ��ζ�Ǿ���ϩ��
��4������2NH4Cl+Ca��OH��2�TCaCl2+2NH3��+2H2O����5��C����6��6CO2+6H2O
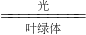 C6H12O6+6O2����7��AC��
C6H12O6+6O2����7��AC����������1�������л��ϳɲ��ϵ��йط�Χ�������
��2������ʳ�������е�Ӫ�����ʷ����ɣ�
��3����������ȼ��ʱ������ͬ�������
��4������ׯ��ȱ�ٵ����ǵ�����Լ��̬���ʲ�����������ʻ��ʹ�õ��й�֪ʶ��ɣ�
��5��������������Խ������к���ʵ������������
��6�����ݹ�����������Ĵ����Ķ�����̼��֪ʶ��д��ѧ����ʽ���ɣ�
��7���������������й���������жϣ�
���������⿼���˽�����Ӧ���Լ���������ص��й�֪ʶ����ɴ��⣬�����������еĿα�֪ʶ���У�Ҫ��ͬѧ�Ǽ�ǿ�Ի���֪ʶ�Ĵ������Ա����Ӧ�ã�

��ϰ��ϵ�д�
�����Ŀ