��Ŀ����
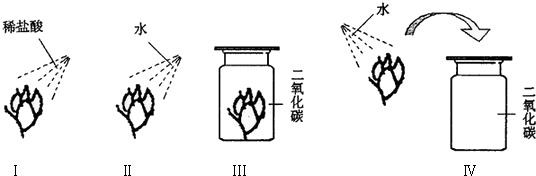
ijͬѧ���������һ��ʵ�飮ȡ�Ķ���ʯ����ҺȾ�ɵ���ɫ�ĸ���ֽ�����ֱ���ͼ����ʵ�飬�ֱ�����ϡ�����ˮ���ش��������⣺
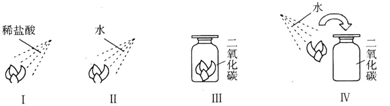
��1���۲쵽ֽ������ɫ�仯�ǣ�
��ֽ����죻��
��2����ͬѧ���еĢ�����ʵ�飬˵��ʲô��
��3����ͬѧ��������Ա�ʵ���Ŀ����ʲô�������������� ��
��4��д��ʵ����з�����Ӧ�Ļ�ѧ����ʽ��
��5��������ʵ�����С��С�ĵؼ��ȣ��۲쵽��������

��1���۲쵽ֽ������ɫ�仯�ǣ�
��ֽ����죻��
ֽ������ɫ
ֽ������ɫ
����ֽ������ɫ������ֽ�����ɫ
ֽ�����ɫ
����2����ͬѧ���еĢ�����ʵ�飬˵��ʲô��
������Һ��ʹʯ����
������Һ��ʹʯ����
����3����ͬѧ��������Ա�ʵ���Ŀ����ʲô�������������� ��
������̼����ʹʯ���ɫ��������̼��ˮ��Һ��ʹʯ����
������̼����ʹʯ���ɫ��������̼��ˮ��Һ��ʹʯ����
����4��д��ʵ����з�����Ӧ�Ļ�ѧ����ʽ��
CO2+H2O�TH2CO3
CO2+H2O�TH2CO3
����5��������ʵ�����С��С�ĵؼ��ȣ��۲쵽��������
ֽ���ɺ�ɫ��Ϊ��ɫ
ֽ���ɺ�ɫ��Ϊ��ɫ
����˵����̼���������
����
��д���йصĻ�ѧ����ʽH2CO3
CO2��+H2O
| ||
H2CO3
CO2��+H2O
��
| ||
��������1����ɫʯ����Һ����������Һ��ɫ��죬ˮ�����ԣ�������̼��ˮ����̼�ᣬ�ݴ˽��з������
��2���Ƚ�����ʵ�飬�������ᣬ������ˮ������ֽ����ɫ�ı仯���з������
��3�����ݢ�ֽ������ɫ�仯�����з������
��4�����ж�����̼��ˮ��Ӧ����̼�ᣬд����Ӧ�Ļ�ѧ����ʽ���ɣ�
��5���������ɵ�̼��ȶ�������ʱ�ֽ⣬���з������
��2���Ƚ�����ʵ�飬�������ᣬ������ˮ������ֽ����ɫ�ı仯���з������
��3�����ݢ�ֽ������ɫ�仯�����з������
��4�����ж�����̼��ˮ��Ӧ����̼�ᣬд����Ӧ�Ļ�ѧ����ʽ���ɣ�
��5���������ɵ�̼��ȶ�������ʱ�ֽ⣬���з������
����⣺��1����ɫʯ����Һ����������Һ��ɫ��죬ˮ�����ԣ���ɫʯ����Һ��ˮ����ɫ��������̼��ˮ����̼�ᣬ���ɵ�̼�������ԣ���ʹֽ�����ɫ��
��2���������ᣬ������ˮ������ɫ����ɫ��˵����ʹʯ����Һ��죬ˮ����ʹ��ɫʯ����Һ��죮
��3�����жԱ�ʵ��˵��������̼�����������ԣ�����ʹʯ���ɫ��������̼��ˮ��Һ�����ԣ���ʹʯ���죮
��4�������ж�����̼��ˮ��Ӧ����̼�ᣬ��Ӧ�Ļ�ѧ����ʽΪ��CO2+H2O�TH2CO3��
��5��̼��ȶ��������ֽ����ɶ�����̼��ˮ��ʹ������ʧ���ʽ�����ʵ�����С��С�ĵؼ��ȣ�ֽ���ɺ�ɫ��Ϊ��ɫ��˵����̼��������ԣ���Ӧ�Ļ�ѧ����ʽΪ��H2CO3
CO2��+H2O��
�ʴ�Ϊ����1��ֽ������ɫ��ֽ�����ɫ����2��������Һ��ʹʯ���죻��3��������̼����ʹʯ���ɫ��������̼��ˮ��Һ��ʹʯ���죻��4��CO2+H2O�TH2CO3����5�����ԣ�H2CO3
CO2��+H2O��
��2���������ᣬ������ˮ������ɫ����ɫ��˵����ʹʯ����Һ��죬ˮ����ʹ��ɫʯ����Һ��죮
��3�����жԱ�ʵ��˵��������̼�����������ԣ�����ʹʯ���ɫ��������̼��ˮ��Һ�����ԣ���ʹʯ���죮
��4�������ж�����̼��ˮ��Ӧ����̼�ᣬ��Ӧ�Ļ�ѧ����ʽΪ��CO2+H2O�TH2CO3��
��5��̼��ȶ��������ֽ����ɶ�����̼��ˮ��ʹ������ʧ���ʽ�����ʵ�����С��С�ĵؼ��ȣ�ֽ���ɺ�ɫ��Ϊ��ɫ��˵����̼��������ԣ���Ӧ�Ļ�ѧ����ʽΪ��H2CO3
| ||
�ʴ�Ϊ����1��ֽ������ɫ��ֽ�����ɫ����2��������Һ��ʹʯ���죻��3��������̼����ʹʯ���ɫ��������̼��ˮ��Һ��ʹʯ���죻��4��CO2+H2O�TH2CO3����5�����ԣ�H2CO3
| ||
�����������ѶȲ��Ǻܴ���ͬѧ�����ö�����̼�Ļ�ѧ���ʽ��н�������������ն�����̼�Ļ�ѧ���ʲ��������������ȷ�����Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ