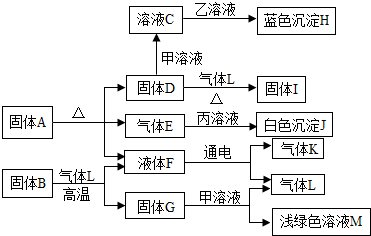
��Ŀ����
���û�ѧ����ʽ��ʾ��
��1�����������̼�ķ���
��2��ʵ������ȡ������ԭ���������ȣ�
��3����¯����ԭ��
��4������ʯ��ˮ�е���̼����Ũ��Һ
��1�����������̼�ķ���
CO2+Ca��OH��2=CaCO3��+H2O
CO2+Ca��OH��2=CaCO3��+H2O
����2��ʵ������ȡ������ԭ���������ȣ�
2H2O2
2H2O+O2��
| ||
2H2O2
2H2O+O2��
��
| ||
��3����¯����ԭ��
Fe2O3+3CO
2Fe+3CO2
| ||
Fe2O3+3CO
2Fe+3CO2
��
| ||
��4������ʯ��ˮ�е���̼����Ũ��Һ
Ca��OH��2+Na2CO3�TCaCO3��+2NaOH
Ca��OH��2+Na2CO3�TCaCO3��+2NaOH
����������д��ѧ����ʽʱ������Ҫȷ����������ݷ�Ӧ���Ԫ����ɺ��ṩ����Ϣ������֪�����Ȼ����ƽ��ע��������
����⣺��1�����ö�����̼������ʹ����ʯ��ˮ����ǵ����ʣ������ó���ʯ��ˮ�����������̼���壮��Ӧʱ��������̼���������Ʒ�Ӧ����̼��Ƴ�����ˮ��
�ʴ�Ϊ��CO2+Ca��OH��2=CaCO3��+H2O��
��2�����������Զ�������Ϊ�����ڲ�����ȵ�����������ˮ���������ʴ�Ϊ��2H2O2
2H2O+O2����
��3��ԭ����һ����̼����������ʯ����Ҫ�ɷ����������������ﻹԭ��������ԭΪ���Ͷ�����̼���ʴ�Ϊ��Fe2O3+3CO
2Fe+3CO2��
��4������������̼���Ʒ�Ӧ����̼��Ƴ������������ƣ��ʴ�Ϊ��Ca��OH��2+Na2CO3�TCaCO3��+2NaOH��
�ʴ�Ϊ��CO2+Ca��OH��2=CaCO3��+H2O��
��2�����������Զ�������Ϊ�����ڲ�����ȵ�����������ˮ���������ʴ�Ϊ��2H2O2
| ||
��3��ԭ����һ����̼����������ʯ����Ҫ�ɷ����������������ﻹԭ��������ԭΪ���Ͷ�����̼���ʴ�Ϊ��Fe2O3+3CO
| ||
��4������������̼���Ʒ�Ӧ����̼��Ƴ������������ƣ��ʴ�Ϊ��Ca��OH��2+Na2CO3�TCaCO3��+2NaOH��
�������������Ĺؼ����ҶԷ�Ӧ������Ȼ���շ���ʽ����д����д������ʽ��

��ϰ��ϵ�д�
 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
�����Ŀ