��Ŀ����
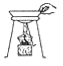
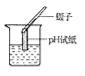
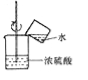
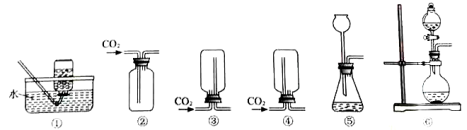
����Ŀ����ͼ��ʵ�����Ʊ�����ij���װ�ú�������

����A�ƶ�����̼����Ҫ��ҩƷ�Ǵ���ʯ��________����Ӧ��ʼ������̼ͨ��___________�У�������ɫ��������Ӧһ��ʱ���н����ɼУ�A��������__________��
��������ʱ����B��_______��ѡ����C������D����������ɷ���װ�á���ˮ���ռ����������ò���Ƭ________��ѡ����ĥɰ�������⻬������һ���ס����ƿ�ڡ�
������������ȼ�յ�������___________��
������0.2mol����طֽ⣬�����������������������ݻ�ѧ����ʽ��ʽ���㣩_____________
���𰸡�����1��ϡ���� ��2������ʯ��ˮ
��3���Թ���Һ�����½�������©����Һ����������һ��ʱ����Һ���룬��Ӧֹͣ
����4��D ��5��ĥɰ
����6������ȼ�գ���������������ɫ���棬�����д̼�����ζ�����壬����
����7��9.6g
����������ʵ������ȡ������̼����Ҫ��ҩƷ�Ǵ���ʯ��ϡ���ᡣ��Ӧ��ʼ������̼ͨ�����ʯ��ˮ�У�������ɫ��������Ӧһ��ʱ���н����ɼУ����ɶ�����̼�����ų���ʹװ������������ѹǿ�����ѹ���������¿ι۲쵽���Թ���Һ�����½�������©����Һ����������һ��ʱ����Һ���룬��Ӧֹͣ����������ʱ������Ӧ��¶��˫����������̫���������ڵ������壬���Խ�B��D������ɷ���װ�á���ˮ���ռ����������ò���Ƭĥɰ��һ���ס����ƿ�ڣ��Է������ݳ���������������ȼ�յ�������_����ȼ�գ��ų�����������������������ɫ���棬������ɫ�д̼�����ζ��������������0.2mol����طֽ⣬��������������Ϊx��
2KClO3 ![]() 2KCl+3O2��
2KCl+3O2��
2 3
0.2mol x
2/0.2mol=3/x x=0.3mol
������������0.3mol��32g/ mol=9.6g

����Ŀ���±���NaCl��KNO3�ڲ�ͬ����ʱ���ܽ�ȣ��ش�������
�¶�/�� | 10 | 20 | 30 | 40 | 50 | 60 | |
�ܽ��/g | NaCl | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 |
KNO3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110.0 | |
��1���������������ܽ�����¶�Ӱ��仯�ϴ����_________��
��2��60��ʱ����ͼʾ������
![]()
A��������_____(����������������������)��Һ��C����Һ����������______g��
��3��50��ʱ�����������ʵı�����Һ��100g���ֱ��������10gˮ���ٻָ���50����ʣ����Һ��������NaCl��Һ_____������������������������С������ KNO3��Һ��