��Ŀ����
![]() ������С���С˼��λͬѧ���һ���������ʵ¼������һ������о������������⡣����������ȷ��0.01��
������С���С˼��λͬѧ���һ���������ʵ¼������һ������о������������⡣����������ȷ��0.01��
[��Ŀ] ��֪: 2KClO3 === 2KCl + 3O2��,��10g����غ�2g�������̻�Ϻ�����Թ��м��ȣ��ռ���������������ֹͣ�������Թ���ȴ���Ƶ��Թ���ʣ����������Ϊ7.2g���������Ȼ��ص�������
��1��С��ܿ�õ���ʽ����10g + 2g –7.2g��������ʽ���ý���� ���ѧʽ������������Ӧ������KCl �������Ƕ��٣�
(2)С˼��������������������Ŀ���������⡣����ͨ���ļ��㣬��֤���ķ��֡�
��3����β��ܸ��������أ�С���С˼��Ϊ������������罫��Ŀ�С�10g����ء���Ϊ��ag����ء����������ʵ��������䣬��a��ȡֵ��Χ�� ��
��1�� O2 7.45g
(2)��ʣ���������=7.45g + 2g =9.45g >7.2g
������Ŀ�д���
�����������= 7.454g + 4.8 g = 12.25g > 10g ������Ŀ�д���
����ݻ�ѧ����ʽ��� ��
(3) 5.2g < a ��8.55g

��ϰ��ϵ�д�
�����Ŀ
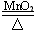 2KCl + 3O2������10g����غ�2g�������̻�Ϻ�����Թ��м��ȣ��ռ���������������ֹͣ�������Թ���ȴ���Ƶ��Թ���ʣ����������Ϊ7.2g���������Ȼ��ص�������
2KCl + 3O2������10g����غ�2g�������̻�Ϻ�����Թ��м��ȣ��ռ���������������ֹͣ�������Թ���ȴ���Ƶ��Թ���ʣ����������Ϊ7.2g���������Ȼ��ص�������