��Ŀ����
��ˮ��һ���������ܼ�����1������淋Ļ�ѧʽΪNH4NO3��ũҵ�ϳ�������Һ��Ϊ �ʣ�����ء���ʹ�ã�����100g 10%���������Һ������Ͳ��ȡ mLˮ������Ҫ�õ���������������ƽ�� ����дһ���������ȣ�
��2������淋�Ħ������Ϊ ��1mol������к� mol��ԭ�ӣ�
��ˮ��һ�ֱ������Դ��
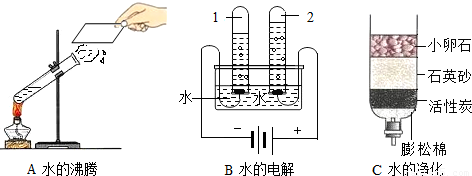
��1����ͼ��ʾ��3��ʵ�飬A��B��ˮ�����仯�ı��������ǣ������ӽǷ����� �� C�о���ˮ�ķ����� ��������B�з�ӦΪ��2H2O
 2H2��+O2�����û�ѧ����ʽ�е�ϵ����������� ����дһ�㣩���Թ�2�ڵõ����������ʾ��ͼΪ ������ͼ����ĸ��ţ���
2H2��+O2�����û�ѧ����ʽ�е�ϵ����������� ����дһ�㣩���Թ�2�ڵõ����������ʾ��ͼΪ ������ͼ����ĸ��ţ��� | ���� | B | C | D |  |
| ��ʾ��ͼ |  |  |  |
���𰸡�������I��1����������淋Ļ�ѧʽΪNH4NO3������������Ϣ������100g 10%���������Һ�����������Ҫˮ������
��2��Ħ�������ĵ�λΪg/mol������ֵ�ϵ��ڸ����ʵ����ԭ����������Է���������
II��1������A�������������仯��B�������ǻ�ѧ�仯������C�о�ˮ�ķ����ǹ��˺����������ۿ����������Ӧ������ﶼ���ɷ��ӹ��ɵģ���ô��ѧ����ʽ����ʾ����Ӧ��������ķ��Ӹ����ȣ�
��2�����ݴ����ܸı��������ʵĻ�ѧ��Ӧ���ʣ��������������ͻ�ѧ���ʲ�������жϣ�
����⣺I��1������淋Ļ�ѧʽΪNH4NO3�����е����еĵ�Ԫ�أ����ڵ��ʣ�����100g 10%���������Һ�����к���������100×10%=10g��������Ҫ��ȡ90mL��ˮ�����ô���Һ��Ҫ����������Ͳ��������ƽ���ձ���
�ʴ�Ϊ������90���ձ���
��2��Ħ�������ĵ�λΪg/mol������ֵ�ϵ��ڸ����ʵ����ԭ����������Է�����������������淋�Ħ������Ϊ80 g/mol��1mol������к�4mol����ԭ�ӣ�
�ʴ�Ϊ��80 g/mol��4��
II��1��A�������������仯��A��ˮ���Ӳ��䣻B�������ǻ�ѧ�仯��B��ˮ���ӱ仯�������������ӣ�C�о�ˮ�ķ����ǹ��˺�������
���ۿ���������������ˮ�����ɷ��ӹ��ɵģ���ˣ������ѧ����ʽ��ʾˮ��ͨ��������£�2��ˮ��������2������Ӻ�1�������ӣ��Թ�2�����ɵ������������������Թ�2�ڵõ����������ʾ��ͼΪB��
�ʴ�Ϊ��A��ˮ���Ӳ��䣬B��ˮ���ӱ仯�������������ӣ����ˣ�2��ˮ��������2������Ӻ�1�������ӣ�B��
��2��þ���Ļ��Ϸ�Ӧʮ�ֻ������μ�����ˮ����Ӧ�������ҽ��У���Ӧ�ٶȴ����ߣ��ɼ�ˮ�ı��˻�ѧ��Ӧ�����ʣ���ˮ���˴������ã�
�ʴ�Ϊ��������
������Ħ�������ĵ�λΪg/mol������ֵ�ϵ��ڸ����ʵ����ԭ����������Է�������������ijһ��������˵������Ħ�������ǹ̶�����ģ������ʵ��������������ʵ�����ͬ�������仯��
��2��Ħ�������ĵ�λΪg/mol������ֵ�ϵ��ڸ����ʵ����ԭ����������Է���������
II��1������A�������������仯��B�������ǻ�ѧ�仯������C�о�ˮ�ķ����ǹ��˺����������ۿ����������Ӧ������ﶼ���ɷ��ӹ��ɵģ���ô��ѧ����ʽ����ʾ����Ӧ��������ķ��Ӹ����ȣ�
��2�����ݴ����ܸı��������ʵĻ�ѧ��Ӧ���ʣ��������������ͻ�ѧ���ʲ�������жϣ�
����⣺I��1������淋Ļ�ѧʽΪNH4NO3�����е����еĵ�Ԫ�أ����ڵ��ʣ�����100g 10%���������Һ�����к���������100×10%=10g��������Ҫ��ȡ90mL��ˮ�����ô���Һ��Ҫ����������Ͳ��������ƽ���ձ���
�ʴ�Ϊ������90���ձ���
��2��Ħ�������ĵ�λΪg/mol������ֵ�ϵ��ڸ����ʵ����ԭ����������Է�����������������淋�Ħ������Ϊ80 g/mol��1mol������к�4mol����ԭ�ӣ�
�ʴ�Ϊ��80 g/mol��4��
II��1��A�������������仯��A��ˮ���Ӳ��䣻B�������ǻ�ѧ�仯��B��ˮ���ӱ仯�������������ӣ�C�о�ˮ�ķ����ǹ��˺�������
���ۿ���������������ˮ�����ɷ��ӹ��ɵģ���ˣ������ѧ����ʽ��ʾˮ��ͨ��������£�2��ˮ��������2������Ӻ�1�������ӣ��Թ�2�����ɵ������������������Թ�2�ڵõ����������ʾ��ͼΪB��
�ʴ�Ϊ��A��ˮ���Ӳ��䣬B��ˮ���ӱ仯�������������ӣ����ˣ�2��ˮ��������2������Ӻ�1�������ӣ�B��
��2��þ���Ļ��Ϸ�Ӧʮ�ֻ������μ�����ˮ����Ӧ�������ҽ��У���Ӧ�ٶȴ����ߣ��ɼ�ˮ�ı��˻�ѧ��Ӧ�����ʣ���ˮ���˴������ã�
�ʴ�Ϊ��������
������Ħ�������ĵ�λΪg/mol������ֵ�ϵ��ڸ����ʵ����ԭ����������Է�������������ijһ��������˵������Ħ�������ǹ̶�����ģ������ʵ��������������ʵ�����ͬ�������仯��

��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�
�����Ŀ
 �������ִ������ҵ������Ӧ�ü�Ϊ�ձ��һ����ϣ�
�������ִ������ҵ������Ӧ�ü�Ϊ�ձ��һ����ϣ���1����֪����ͬ�������£������Ļ��Խǿ���������ᷴӦ�������ݣ����������ٶȾ�Խ�죮Al��Cu��Fe���ֽ�����ϡ������ķ�Ӧ��������ͼ��ʾ��
����ͼ��Y�������Ľ�����
��Al��Cu��Fe���ֽ����Ļ����ǿ������˳��Ϊ
������һ�ֻ��ý���������������ȴ�н�ǿ�Ŀ���ʴ�ԣ���ԭ����
��2�������Ŀ����������������������������ı�־��
���ҹ��Ŵ���¯��ʯ��ZnCO3������ͭ��Cu2O����ľ̿�ۻ�ϼ�����800�����ң����ɵõ���ƽ�������Ƶġ�ҩ�𡱣�
I�����������������Ƶ������Ļƽ�Au������Ϊ
II����ҩ����
���������²����ᡢ�Ӧ�����������ܣ�����Ϊ��δ�����������ɷ��Ѵ�������ȡ�����ѵ���Ҫ���չ������£�
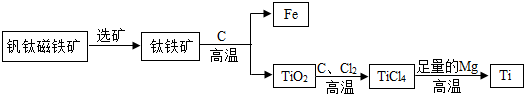
�ڸ�����������Mg��Ӧ�û����ɽ���Ti���÷�Ӧ�Ļ�ѧ����ʽΪ��
�������������еõ��Ľ������л��������������ʣ��ɼ���
��3����֪ij������ĩ�г�����Al�����һ������Fe��Cu��Ϊ֤��Fe��Cu�Ĵ��ڲ��ⶨ����Al������������ij��ѧ��ȤС���ͬѧչ�������µ�ʵ��̽����
�������ߣ�Al������������Һ��Ӧ��������ˮ��ƫ�����ƺ���������Ӧ�Ļ�ѧ����ʽΪ��2Al+2H2O+2NaOH=2NaAlO2+3H2������Fe��Cu��������������Һ��Ӧ��
�������֤��������ĩ�д���Fe��Cu��ʵ����ƣ�
| ʵ����� | ʵ������ | ���� |
| ��ȡ�����Ľ�����ĩ���Թ��У����������� |
����ȥ�� | |
| ���Թܾ��ã���ȥ�ϲ���Һ������������ϡ���ᣮ | ֤�������� | |
| ���Թܾ��ã���ȥ�ϲ���Һ�����ϴ��ʣ����� | ʣ�������Ϻ�ɫ | ֤������ͭ |
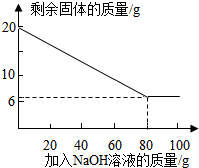 ��Ϊ̽���ý�����ĩ��Al������������ȡ20 g�ý�����ĩ����100 g����������Һƽ���ֳ�5�����μ��룬��ַ�Ӧ���˳����壬����ϴ�ӡ����������ʵ������еõ��IJ���������ͼ�����£�
��Ϊ̽���ý�����ĩ��Al������������ȡ20 g�ý�����ĩ����100 g����������Һƽ���ֳ�5�����μ��룬��ַ�Ӧ���˳����壬����ϴ�ӡ����������ʵ������еõ��IJ���������ͼ�����£�| ��NaOH��Һ�Ĵ��� | ��һ�� | �ڶ��� | ������ | �� |
| ʣ����������/g | 16.5 | n | 9.5 | �� |
�ý�����ĩ��Al����������Ϊ
����ʽ���㣺��������������Һ��������������Ϊ���٣�
