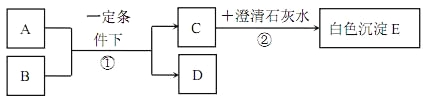
��Ŀ����
����Ŀ����֪ij�������Ƶõ�Na2CO3���׳ƣ�����ֲ�Ʒ�к��������Ȼ��ƣ�Ϊ�˲ⶨ�ֲ�Ʒ�д���Ĵ��ȣ���������ͼװ�õ�ʵ�飺����Eװ���еı���NaHCO3��Һ��Ϊ�˳�ȥ������̼�����е��Ȼ��⣻�����ķ�ӦΪNaHCO3+HCl�TNaCl+H2O+CO2����
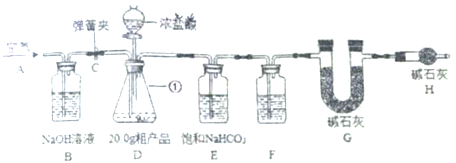
�����Ӻ�װ�ã���������ԣ���20g�ֲ�Ʒ������ƿ�У�����������ˮ�ܽ⣬�õ�������Һ��
�ڴ��ɼ�C����A������ͨ��һ��ʱ�������
�۳��� G��������
�ܹرյ��ɼ�C�������μ�Ũ����ֱ��D��������ð����
�ݴ��ɼ�C���ٴλ���ͨ��һ��ʱ�������
���ٴγ���G����������ǰ������������Ϊ4.8g��
��ش���������
��1��д�������ٵ����� ��F�е��Լ�ӦΪ ��
��2��Bװ�õ������� ��Bװ���з�����Ӧ�Ļ�ѧ����ʽΪ ��
��3���ڲ�����У�A������ͨ��һ��ʱ�������Ŀ���� ��
��4����û��Hװ�ã��ⶨ��Na2CO3������������ ���ƫ����ƫС���������䡱����
��5������ȤС��ָ����ʦ��Ϊ�÷�����Щ��������ָ��ͬѧ�Ǹ����˹��е�һ���Լ���ȥ����һ��װ�ú�ʵ��õ��˸��ƣ������20.0g�ֲ�Ʒֻ�ܲ���4.4gCO2������Ϊ��������Լ��� ��ԭʵ����ʵ��ֵ4.8g����ȷֵ4.4gƫ���ԭ���ǣ������������ȷ�� �����ݼ��㣺������ȷֵ4.4g�������Ʒ��Na2CO3������������
���𰸡���1����ƿ�� Ũ������2����ȥ�����еĶ�����̼��2NaOH+CO2�TNa2CO3+H2O����3���ų�װ���п�����4��ƫ����5��ϡ���ᣮŨ�����лӷ��ԣ�����������̼�к�HCl������̼��������Һ��Ӧ�������˶�����̼��53%��
��������
��1�������ٵ�������ƿ����ΪŨ����������ˮ��������F�е��Լ�ӦΪŨ���
��2������������Һ�����տ����еĶ�����̼����Bװ���з�����Ӧ�Ļ�ѧ����ʽΪ2NaOH+CO2�TNa2CO3+H2O��
��3����Aװ����ͨ��������Խ�װ���е������ž�����ֹ����ʵ�������ۣ�
��4��Hװ���ܷ�ֹ�����еĶ�����̼��ˮ��������Gװ�ã���û��Hװ�ã���ⶨ�Ķ�����ֵ̼ƫ�ߣ����������������ƫ��
��5��װ��D��Ũ����ӷ������Ȼ�����װ��E��NaHCO3��Ӧ����������̼��ʹ������̼��ֵƫ��Ϊ��ʹʵ������ȷ��ֹ���ţ�����Ũ���ỻ��ϡ���ᣬ
��̼���Ƶ�����Ϊx��
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 44
x 4.4g
��![]() ����ã�x=10.6g��
����ã�x=10.6g��
̼���Ƶ���������Ϊ��![]() ��100%=53%��
��100%=53%��
��̼���Ƶ�����������53%��

����Ŀ��ͼʾ���Լ�����ֱ�۵ı���һЩ��Ϣ���ǻ�ѧѧϰ��һ����Ҫ�ֶΣ���ϸ��ͼ������ͼʾ������и��⣮

��1��ͼ1�DZ�ʾ ��
��2��ͼ2��Ԫ�����ڱ����Ԫ�ص�ijЩ��Ϣ���ݴ˿�֪��믵����ԭ�������� ��
��3��ͼ3����ԭ�ӵĽṹʾ��ͼ�����У�x= ����ԭ���ڻ�ѧ��Ӧ������ ����á���ʧ�������ӣ�����ؿ��к������Ľ���Ԫ���γɻ�����Ļ�ѧʽΪ ��
��4���±�ΪԪ�����ڱ���ijһ����Ԫ�ص�ԭ�ӽṹʾ��ͼ����ش��������⣺
Ԫ������ | �� | þ | �� | �� | �� | �� | �� | � |
Ԫ�ط��� | Na | Mg | Al | Si | P | S | Cl | Ar |
ԭ�ӽṹʾ��ͼ |
|
|
|
|
|
|
|
|
��д����16��Ԫ�ص����ӷ��� ��
������Ԫ�������ڱ��д���ͬһ���ڵ�ԭ���� /span> ��