��Ŀ����
 ��2009?�Թ���ij��Ƭ�ı�ǩ���ң���֪�˸�Ƭ�ɷ���ֻ��̼��ƺ��и�Ԫ�أ�
��2009?�Թ���ij��Ƭ�ı�ǩ���ң���֪�˸�Ƭ�ɷ���ֻ��̼��ƺ��и�Ԫ�أ���1����ͨ������˵���˱�ǩ�еĺ������Ǵ���ģ�
��2��Ϊ�ⶨ����ʵ�ĺ�������С��ÿ��ȡ10Ƭ��Ƭ�����ѳ����ĺ�����������ձ��У������Ļ�ѧ��Ӧ�ǣ�CaCO3+2HCl�TCaCl2+H2O+CO2������ַ�Ӧ���ٳ�ȡ�ձ���ʣ�������������С����������ʵ�飬��������
| ���ʵ����� | ��һ�� | �ڶ��� | ������ | ƽ��ֵ |
| ��Ӧǰ���ձ�+���� | 22 g | 22 g | 22 g | 22 g |
| 10Ƭ��Ƭ | 8 g | 8 g | 8 g | 8 g |
| ��Ӧ���ձ�ʮʣ���� | 26.7 g | 26.5 g | 26.9 g | 26.7g |
������ʽ����ÿƬ�˸�Ƭ�ĺ������������鳧������ı�ǩ��
���𰸡�������ȡ�������ݵ�ƽ��ֵ�����ݱ�ǩ֪�����ɶ�����̼������Ϊ��Ӧǰ���������ٸ��ݷ���ʽ�ж�����̼��̼��Ƶ�������ϵ���
10Ƭ��Ƭ��̼��Ƶ��������Ӷ�����ÿƬ�˸�Ƭ��̼��Ƶ�������
����˸�Ƭ��ÿƬ�˸�Ƭ�ĺ����������ǩ���ݱȽϷ���������֪����θı�ǩ��
����⣺��1����Ʒ��Ϊ��̼��ƣ�̼��Ƶ���Է�������Ϊ100����Ԫ�ص���������Ϊ40 100×100%=40%��
ÿƬ������Ϊ��40g 50×40%=0.32g
��˱�ƷÿƬ�����ܺ���0.75g���˱�ǩ�еĺ���������
��2���裺10Ƭ��Ƭ��̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 3.3g
x=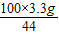 =7.5g
=7.5g
ÿƬ�˸�Ƭ��̼���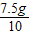 =0.75g
=0.75g
��̼��ƺ�����������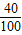 ×100%=40%
×100%=40%
ÿƬ�˸�Ƭ�ĺ�������0.75g×40%=0.3g
�𣺢���˱�ƷÿƬ�����ܺ���0.75g���˱�ǩ�еĺ���������
�ڽ���������ÿƬ����0.75g����Ϊ��ÿƬ����0.3g����
��������ǩ���ǽ������п��������ֵ������⣬��ῴ����ǩ���ݣ��Ż���ȷ���
10Ƭ��Ƭ��̼��Ƶ��������Ӷ�����ÿƬ�˸�Ƭ��̼��Ƶ�������
����˸�Ƭ��ÿƬ�˸�Ƭ�ĺ����������ǩ���ݱȽϷ���������֪����θı�ǩ��
����⣺��1����Ʒ��Ϊ��̼��ƣ�̼��Ƶ���Է�������Ϊ100����Ԫ�ص���������Ϊ40 100×100%=40%��
ÿƬ������Ϊ��40g 50×40%=0.32g
��˱�ƷÿƬ�����ܺ���0.75g���˱�ǩ�еĺ���������
��2���裺10Ƭ��Ƭ��̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 3.3g
x=
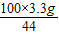 =7.5g
=7.5gÿƬ�˸�Ƭ��̼���
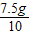 =0.75g
=0.75g��̼��ƺ�����������
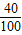 ×100%=40%
×100%=40%ÿƬ�˸�Ƭ�ĺ�������0.75g×40%=0.3g
�𣺢���˱�ƷÿƬ�����ܺ���0.75g���˱�ǩ�еĺ���������
�ڽ���������ÿƬ����0.75g����Ϊ��ÿƬ����0.3g����
��������ǩ���ǽ������п��������ֵ������⣬��ῴ����ǩ���ݣ��Ż���ȷ���

��ϰ��ϵ�д�
 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�
�����Ŀ
 ��2009?�Թ���ij��Ƭ�ı�ǩ���ң���֪�˸�Ƭ�ɷ���ֻ��̼��ƺ��и�Ԫ�أ�
��2009?�Թ���ij��Ƭ�ı�ǩ���ң���֪�˸�Ƭ�ɷ���ֻ��̼��ƺ��и�Ԫ�أ���1����ͨ������˵���˱�ǩ�еĺ������Ǵ���ģ�
��2��Ϊ�ⶨ����ʵ�ĺ�������С��ÿ��ȡ10Ƭ��Ƭ�����ѳ����ĺ�����������ձ��У������Ļ�ѧ��Ӧ�ǣ�CaCO3+2HCl�TCaCl2+H2O+CO2������ַ�Ӧ���ٳ�ȡ�ձ���ʣ�������������С����������ʵ�飬��������
| ���ʵ����� | ��һ�� | �ڶ��� | ������ | ƽ��ֵ |
| ��Ӧǰ���ձ�+���� | 22 g | 22 g | 22 g | 22 g |
| 10Ƭ��Ƭ | 8 g | 8 g | 8 g | 8 g |
| ��Ӧ���ձ�ʮʣ���� | 26.7 g | 26.5 g | 26.9 g | 26.7g |
������ʽ����ÿƬ�˸�Ƭ�ĺ������������鳧������ı�ǩ��
 ��2009?�Թ���ij��Ƭ�ı�ǩ���ң���֪�˸�Ƭ�ɷ���ֻ��̼��ƺ��и�Ԫ�أ�
��2009?�Թ���ij��Ƭ�ı�ǩ���ң���֪�˸�Ƭ�ɷ���ֻ��̼��ƺ��и�Ԫ�أ���1����ͨ������˵���˱�ǩ�еĺ������Ǵ���ģ�
��2��Ϊ�ⶨ����ʵ�ĺ�������С��ÿ��ȡ10Ƭ��Ƭ�����ѳ����ĺ�����������ձ��У������Ļ�ѧ��Ӧ�ǣ�CaCO3+2HCl�TCaCl2+H2O+CO2������ַ�Ӧ���ٳ�ȡ�ձ���ʣ�������������С����������ʵ�飬��������
| ���ʵ����� | ��һ�� | �ڶ��� | ������ | ƽ��ֵ |
| ��Ӧǰ���ձ�+���� | 22 g | 22 g | 22 g | 22 g |
| 10Ƭ��Ƭ | 8 g | 8 g | 8 g | 8 g |
| ��Ӧ���ձ�ʮʣ���� | 26.7 g | 26.5 g | 26.9 g | 26.7g |
������ʽ����ÿƬ�˸�Ƭ�ĺ������������鳧������ı�ǩ��

