��Ŀ����
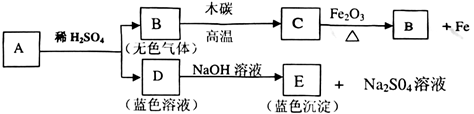
��2010���㽭̨�ݣ������ӹ�ʱ��������̼������̼��Ļ�ѧ�ɷ���ʲô��ͨ�����ϻ�Ϥ���������п��ܺ���Na2SO����Ca(OH)2��MgCl2��NaCl�ȣ�Ϊ��֤��ɷ֣�С�½���������ʵ�飺
(1)���������������̼���Һ�п϶�û�� ��
(2)д��ʵ��2�з����Ļ�ѧ��Ӧ����ʽ�� ��
(3)д��ʵ��3�г��ֵİ�ɫ�������ʵĻ�ѧʽ ��
| ʵ�� | ʵ����� | ʵ������ |
| 1 | ȡ�������̼���Һ���Թ��У����뼸����ɫ��̪��Һ | ��Ϊ��ɫ��Һ |
| 2 | ��ȡ�������̼���Һ���Թ��У�����������Ba(NO3)2��Һ��ϡHNO3 | �а�ɫ���� |
| 3 | ȡʵ��2�е�������Һ���Թ��У�����������AgNO3��Һ��ϡHNO3 | �а�ɫ���� |
(2)д��ʵ��2�з����Ļ�ѧ��Ӧ����ʽ�� ��
(3)д��ʵ��3�г��ֵİ�ɫ�������ʵĻ�ѧʽ ��
(l)Ca(OH)2�����������ƣ� (2) Ba(NO3)2 +Na2SO4 =BaSO4�� +2 NaNO3 (3) AgCl
��1������������Һ�Լ��ԣ���ʹ��̪��Һ��죻ȡ�������̼���Һ���Թ��У����뼸����ɫ��̪��Һ����Һ����ɫ��˵�������������ƣ��ʴ�Ϊ��Ca��OH��2�����������ƣ���
��2����ȡ�������̼���Һ���Թ��У�����������Ba��N03��2��ϡHNO3��˵�����ɲ�����ˮ��ϡ��������ᱵ�������Ӷ�˵�����̼��к��������ƣ������ƺ����ᱵ��Ӧ�������ᱵ�����������ƹʴ�Ϊ��Ba��N03��2+Na2S04=BaS04��+2 NaN03��
��3��ȡʵ��2�е�������Һ���Թ��У�����������AgNO3��Һ��ϡHNO3���а�ɫ������˵�������Ȼ���������˵����Һ�к��������ӣ��ʴ�Ϊ��AgCl��
���������⿼����ָʾ���ı�ɫ������������ӵļ��鷽����Ҫ��ͬѧ���ڽ��ʱҪ����ϸ�£��������֪ʶ��
��2����ȡ�������̼���Һ���Թ��У�����������Ba��N03��2��ϡHNO3��˵�����ɲ�����ˮ��ϡ��������ᱵ�������Ӷ�˵�����̼��к��������ƣ������ƺ����ᱵ��Ӧ�������ᱵ�����������ƹʴ�Ϊ��Ba��N03��2+Na2S04=BaS04��+2 NaN03��
��3��ȡʵ��2�е�������Һ���Թ��У�����������AgNO3��Һ��ϡHNO3���а�ɫ������˵�������Ȼ���������˵����Һ�к��������ӣ��ʴ�Ϊ��AgCl��
���������⿼����ָʾ���ı�ɫ������������ӵļ��鷽����Ҫ��ͬѧ���ڽ��ʱҪ����ϸ�£��������֪ʶ��

��ϰ��ϵ�д�
 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�
�����Ŀ