��Ŀ����
 ��2006?üɽ��С����ʵ���ҷ���һ�ֺ�ɫ��ĩ��һƿ��ǩ�������ɫ��Һ����ͼ��ʾ������ȡ������ɫ��ĩ����Һ��ϣ��������������˴�����ɫ�����ݣ�
��2006?üɽ��С����ʵ���ҷ���һ�ֺ�ɫ��ĩ��һƿ��ǩ�������ɫ��Һ����ͼ��ʾ������ȡ������ɫ��ĩ����Һ��ϣ��������������˴�����ɫ�����ݣ���1����������������ʲô�������д��룮С�ѵIJ�������ǣ�
��
H2O2
H2O2
��MnO2
MnO2
����
H2SO4
H2SO4
������
����
����2���������С��ͬѧ���ʵ�飬��֤�����������ȷ�ģ�
| ʵ�鲽�� | ʵ���¼ | ʵ����� |
| ��ȡ������ɫ��ĩ���Թ��У��μ�������ɫ��Һ �� �ռ�һƿ���Թܣ����壬�������ǵ�ľ������ƿ���Թܣ��� �ռ�һƿ���Թܣ����壬�������ǵ�ľ������ƿ���Թܣ��� | �������ݲ��� �� �����ǵ�ľ����ȼ �����ǵ�ľ����ȼ | ������������ ���� ���� ��ԭ����ٳ����� |
�������������ʵ���ɫ��ȱʧ��ǩ�в������֣��ɲ����ɫ��ĩ�Ƕ������̣���Һ��H2O2��Һ������������ʵ�鲽�裮
����𣺽⣺�ɱ�ǩ�в����ġ�H2�����Բ������ҺΪ���˫��ˮ��Ȼ���ٸ��ݼ����ɫ��ĩ���������������������Ʋ����ɫ��ĩΪ�������̣�����ɫ��ҺΪ˫��ˮ��ʵ�鲽���жԲ�������������Ӧ���ô����ǵ�ľ�����м��飮
�ʴ�Ϊ����1��MnO2��H2O2��Һ��H2SO4�����ۣ� ��2��
�����ռ�һ�Թ������ƽ��ƾ��ƵĻ��棨���Ȼ�ŵ�ľ���ƽ��Թܿڣ���������������������ȼ�գ�����ʵ���ɫ����H2
�ʴ�Ϊ����1��MnO2��H2O2��Һ��H2SO4�����ۣ� ��2��
| ʵ�鲽�� | ʵ���¼ | ʵ����� |
| ��ȡ������ɫ��ĩ���Թ��У��μ�������ɫ��Һ�� ���ô����ǵ�ľ�������Թܿڣ� | �������ݲ��� ��ľ����ȼ | ������������������ ԭ��������� |
���������⿼�鳣�����ʵ����ʣ�Ҫ�����Ǹ������ʵ����ʡ���ѧʽ�ͷ�Ӧ�����ƶϳ����ʣ������г�������ļ��飮

��ϰ��ϵ�д�
�����Ŀ
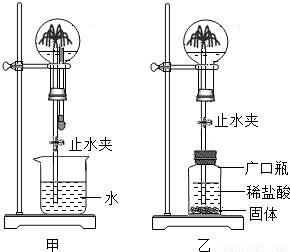
 ��2006?üɽ����1��ij��ѧ�С���ʵ������ȡ����ķ���װ�ý���̽������ͼ�ǸĽ����ʵ��װ��ͼ��
��2006?üɽ����1��ij��ѧ�С���ʵ������ȡ����ķ���װ�ý���̽������ͼ�ǸĽ����ʵ��װ��ͼ��