��Ŀ����
ijͬѧȥ������ɽ�羰�����棬ȡ�������ɿ��ʯ��Ʒ�������������·�������Ʒ��̼��Ƶ������������м�⣺ȡ����ʯ��ʯ��Ʒ6g����40gϡ������Ĵμ��룬�������������������£���֪ʯ��ʯ��Ʒ�к��е����ʲ�����ˮ���������ᷴӦ����
| ����ϡ����Ĵ��� | 1 | 2 | 3 | 4 |
| ����ϡ���������/g | 10 | 10 | 10 | 10 |
| ʣ����������/g | 4.0 | m | 0.6 | 0.6 |
��1��6gʯ��ʯ��Ʒ�к��е�����Ϊ______g��
��2��m=______��
��3����Ʒ��̼��Ƶ�����������
��4������ϡ�������������
��5������ȡ��ʯ��ʯ��Ʒ10g����������g������������ϡ�����ǡ����ȫ��Ӧ����Ӧ��������Һ�����ʵ�����������
�⣺��1���ɷ����Լ��Ƚϵ����κ͵��Ĵε�����6g��Ʒ�����ʵ�������0.6g��
��2����Ʒ��6g�������һ��10g�����ʱ��ʣ�������4g��˵����Ӧ����̼��Ƶ�����Ϊ2g�����ݺ�������ݿ�֪��ʱ̼���Ӧ����ʣ�࣬Ҳ����10g�����2g̼�����ǡ����ȫ��Ӧ�����Եڶ����ټ�10g���ᣬ��Ӧ����̼��ƻ�������2g������ʣ��2g���壬���ݺ���Ĺ���������֪���ڶ���Ӧ��Ҳ�Ƿ�Ӧ��2g��ʣ��2g����m=2g��
��3���������ʵ�������0.6g��������Ʒ��CaCO3����������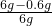 ��100%=90%��
��100%=90%��
��4�������������Ϊx����
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73
2g x
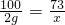
x=1.46 g��
�������������������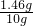 ��100%=14.6%��
��100%=14.6%��
��2������ȡ��ʯ��ʯ��Ʒ10g����̼��Ƶ�������10g��90%=9g��
��������ɸ�����������ϡ����������y�������Ȼ��Ƶ�������z�����ɶ�����̼��������m
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73 111 44
9g y��14.6% z m
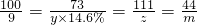
y=45g z=9.99g m=3.96g
��Ӧ����Һ��������9g+45g-3.96g=50.04g�����ʵ�����������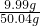 ��100%��19.96%
��100%��19.96%
�ʴ�Ϊ����1��0.66g����2��2��
��3����Ʒ��̼��Ƶ���������Ϊ90%��
��4������ϡ�������������Ϊ14.6%
��5����Ӧ��������Һ�����ʵ���������Ϊ19.96%��
�������������ڷ������ݵ���Ч�ԣ���Ʒ��6g�������һ��10g�����ʱ��ʣ�������4g��˵����Ӧ����̼��Ƶ�����Ϊ2g�����ݺ�������ݿ�֪��ʱ̼���Ӧ����ʣ�࣬Ҳ����10g�����2g̼�����ǡ����ȫ��Ӧ�����Եڶ����ټ�10g���ᣬ��Ӧ����̼��ƻ�������2g������ʣ��2g���壬���ݺ���Ĺ���������֪���ڶ���Ӧ��Ҳ�Ƿ�Ӧ��2g��ʣ��2g����m=2g�������μ���10g���ᣬ�Ǵ�ʣ���2g�������Ϊ0.6g��˵����ʱ̼����Ѿ���ȫ��Ӧ������0.6g����̼��Ƴɷ֣���Ŀ��Ҫ���㷴Ӧ������Һ�����ʵ���������������Ҫ�����ʺ���Һ����������
����������ס��¼�������ڷ����ű仯�����ݣ��������ݱ仯��ԭ���仯�Ĺ��ɣ����Ƿ���ʵ�����ݵ�һ�ֳ��÷�����Ҳ��������Ľ���ؼ���
��2����Ʒ��6g�������һ��10g�����ʱ��ʣ�������4g��˵����Ӧ����̼��Ƶ�����Ϊ2g�����ݺ�������ݿ�֪��ʱ̼���Ӧ����ʣ�࣬Ҳ����10g�����2g̼�����ǡ����ȫ��Ӧ�����Եڶ����ټ�10g���ᣬ��Ӧ����̼��ƻ�������2g������ʣ��2g���壬���ݺ���Ĺ���������֪���ڶ���Ӧ��Ҳ�Ƿ�Ӧ��2g��ʣ��2g����m=2g��
��3���������ʵ�������0.6g��������Ʒ��CaCO3����������
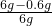 ��100%=90%��
��100%=90%����4�������������Ϊx����
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73
2g x
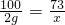
x=1.46 g��
�������������������
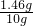 ��100%=14.6%��
��100%=14.6%����2������ȡ��ʯ��ʯ��Ʒ10g����̼��Ƶ�������10g��90%=9g��
��������ɸ�����������ϡ����������y�������Ȼ��Ƶ�������z�����ɶ�����̼��������m
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73 111 44
9g y��14.6% z m
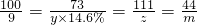
y=45g z=9.99g m=3.96g
��Ӧ����Һ��������9g+45g-3.96g=50.04g�����ʵ�����������
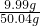 ��100%��19.96%
��100%��19.96%�ʴ�Ϊ����1��0.66g����2��2��
��3����Ʒ��̼��Ƶ���������Ϊ90%��
��4������ϡ�������������Ϊ14.6%
��5����Ӧ��������Һ�����ʵ���������Ϊ19.96%��
�������������ڷ������ݵ���Ч�ԣ���Ʒ��6g�������һ��10g�����ʱ��ʣ�������4g��˵����Ӧ����̼��Ƶ�����Ϊ2g�����ݺ�������ݿ�֪��ʱ̼���Ӧ����ʣ�࣬Ҳ����10g�����2g̼�����ǡ����ȫ��Ӧ�����Եڶ����ټ�10g���ᣬ��Ӧ����̼��ƻ�������2g������ʣ��2g���壬���ݺ���Ĺ���������֪���ڶ���Ӧ��Ҳ�Ƿ�Ӧ��2g��ʣ��2g����m=2g�������μ���10g���ᣬ�Ǵ�ʣ���2g�������Ϊ0.6g��˵����ʱ̼����Ѿ���ȫ��Ӧ������0.6g����̼��Ƴɷ֣���Ŀ��Ҫ���㷴Ӧ������Һ�����ʵ���������������Ҫ�����ʺ���Һ����������
����������ס��¼�������ڷ����ű仯�����ݣ��������ݱ仯��ԭ���仯�Ĺ��ɣ����Ƿ���ʵ�����ݵ�һ�ֳ��÷�����Ҳ��������Ľ���ؼ���

��ϰ��ϵ�д�
�����Ŀ
���±���Ũ�����Լ�ƿ�ϱ�ǩ�����ݣ���ش�
| ���ᣨ�������� ��ѧʽ_________ ������������98% �ܶ�1.84g/cm3 |
��2������������ᶼ��ʹ��ɫʯ����Һ��죬����Һ�к��н϶��______��д��ѧ���ţ���
��3����Ҫ����19.6%��ϡ����200g����Ҫ����Ũ����______mL��
��4��ʹ��һ��ʱ���Ũ�����������������______������ڡ���С�ڡ���98%��ԭ����______��
��5����ͼ���ձ�������ע��Ũ���ᣬ��ῴ����������______��

��ֱ���һ���Լ���ȥ���и������е����ʣ�������ѡ�Լ�������ǡ����ȫ��Ӧ����
| ���� | ���� | �����������Լ����ѧʽ�� | |
| ��1�� | CO | CO2 | ______ |
| ��2�� | Cu | Fe | ______ |
| ��3�� | FeCl2 | CuCl2 | ______ |
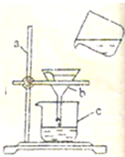 ��ͼ��ijͬѧ������ȥˮ�е���ɳ��ʵ��ʱ�Ĺ���װ��ͼ���Իش�
��ͼ��ijͬѧ������ȥˮ�е���ɳ��ʵ��ʱ�Ĺ���װ��ͼ���Իش�