��Ŀ����
ij��ѧ��ȤС���ڻ��ʳ����з���ij�ּط���Ʒ����ɷ�Ϊ̼��ء��Ȼ��ؼ�����������Ԫ�ص����������ʣ�Ϊ�ⶨ�üط���Ʒ��̼��������ͼ�Ԫ�ص�����������С��������ͬѧ����������ʵ�飺�ٳ�ȡ15g�ط���Ʒ��������ƿ������Ϊ50g ���У�ͬʱ��װ��ҩƷ����ƿ���ڵ�����ƽ������������ͼ��ʾ��
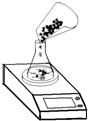
��ֱ������ƽ�ϵ���ƿ�м���20gϡ���ᣬ����ÿ��30������ƿ�м���20gϡ���ᣬ��������ǰ��ƽ�ϵ�ʾ����¼����
| ʱ��/s | 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 |
| ʾ��/g | 84.45 | 103.90 | 123.35 | 142.80 | 162.80 | 182.80 | 252.00 | 222.80 |
��ȡ��Һ������������������õ����������������ᾧˮ��14.9g
���⣺
��1���ڢڲ�ʵ���м�¼�����ݲ���������ʱ��Ϊ
��2��ͬѧ����Ϊ�ⶨ������̼������ʵ�鷽����������
��3������ʵ���֪��Ʒ��̼�������Ϊ���٣�
��4������Ʒ�м�Ԫ����������Ϊ���٣�
��������1����Ӧǰ�������������ʵ�����������ƽʾ���IJ�ֵ���Ƕ�����̼���������ֱ�����ÿһ�η�Ӧ���ɵĶ�����̼������������Ϳ��Է������������ݲ�������Ȼ��������ݻ��Ʒ�Ӧͼ��
��2���ⶨ������̼�������������Ȳ������������Ȼ������ܶ����������Ҳ����������������Һ���ն�����̼����������������Һ�������仯������ó����ۣ�
��3������������Ʒ��ȫ��Ӧ�����ɵĶ�����̼������������̼��غ����ᷴӦ�Ļ�ѧ����ʽ���㼴�������Ʒ��̼��ص�������
��4�����������غ㶨�ɿ�֪����Һ���Ȼ����м�Ԫ�ص���������ԭ��Ʒ�м�Ԫ�ص������������Ȼ��ص��������Ȼ����м�Ԫ�ص����������Ϳ������Ԫ�ص�������Ȼ�������Ʒ�������������Ԫ�ص�����������
��2���ⶨ������̼�������������Ȳ������������Ȼ������ܶ����������Ҳ����������������Һ���ն�����̼����������������Һ�������仯������ó����ۣ�
��3������������Ʒ��ȫ��Ӧ�����ɵĶ�����̼������������̼��غ����ᷴӦ�Ļ�ѧ����ʽ���㼴�������Ʒ��̼��ص�������
��4�����������غ㶨�ɿ�֪����Һ���Ȼ����м�Ԫ�ص���������ԭ��Ʒ�м�Ԫ�ص������������Ȼ��ص��������Ȼ����м�Ԫ�ص����������Ϳ������Ԫ�ص�������Ȼ�������Ʒ�������������Ԫ�ص�����������
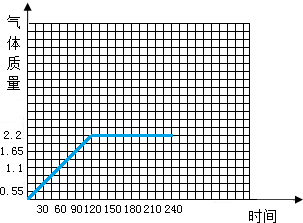
����⣺��1���ɱ������ݿ��Լ����ÿһʱ��ʱ���������������
30sʱ���ɶ�����̼������Ϊ��15g+50g+20g-84.45g=0.55g
60sʱ���ɶ�����̼������Ϊ��15g+50g+20g+20g-103.9g=1.1g
90sʱ���ɶ�����̼������Ϊ��15g+50g+20g+20g+20g-123.35g=1.65g
120sʱ���ɶ�����̼������Ϊ��15g+50g+20g+20g+20g+20g-142.80g=2.2g
150sʱ���ɶ�����̼������Ϊ��15g+50g+20g+20g+20g+20g+20g-162.80g=2.2g
180sʱ���ɶ�����̼������Ϊ��15g+50g+20g+20g+20g+20g+20g+20g-182.80g=2.2g
210sʱ���ɶ�����̼������Ϊ��15g+50g+20g+20g+20g+20g+20g+20g+20g-252.00g=-37g
240sʱ���ɶ�����̼������Ϊ��15g+50g+20g+20g+20g+20g+20g+20g+20g+20g-222.80g=2.2g
�ɼ������ݿ�֪����120sʱ��Ӧֹͣ�����ɵ����屣����2.2g���䣬����210sʱ���ݲ��������������岻����Ϊ��ֵ��ҲӦ�ü������������Ϊ2.2g��
�ʴ�Ϊ��210��
ͼ�����£�
��2��Ҫ������ɶ�����̼����������ͨ���ⶨ������̼�������Ȼ���ٸ����ܶ����������Ҳ����������������Һ���ն�����̼���壬ͨ������������Һ�����ı仯������������̼��������
�ʴ�Ϊ��ͨ������������̼�������������Ķ�����̼���ܶȣ������õ�������̼��������
����������������Һ���ն�����̼���壬ͨ���������������Һ�����仯�õ�������̼������������
��3���������Ϸ�����֪��Ʒ�������ַ�Ӧ�����ɵĶ�����̼��������Ϊ2.2g��
����Ʒ��̼��ص�����Ϊx
K2CO3+2HCl=2KCl+H2O+CO2��
138 44
x 2.2g
=
x=6.9g
����Ʒ��̼��ص�����Ϊ6.9g��
��4������Ʒ�������ַ�Ӧ����ˣ���Һ��ֻ�����Ȼ��أ�
���Ȼ��ص�����Ϊ14.9g
���Ԫ�ص�����=14.9g��
��100%=7.8g
����Ʒ�м�Ԫ�ص���������=
��100%=52%
����Ʒ�м�Ԫ�ص�����������52%��
30sʱ���ɶ�����̼������Ϊ��15g+50g+20g-84.45g=0.55g
60sʱ���ɶ�����̼������Ϊ��15g+50g+20g+20g-103.9g=1.1g
90sʱ���ɶ�����̼������Ϊ��15g+50g+20g+20g+20g-123.35g=1.65g
120sʱ���ɶ�����̼������Ϊ��15g+50g+20g+20g+20g+20g-142.80g=2.2g
150sʱ���ɶ�����̼������Ϊ��15g+50g+20g+20g+20g+20g+20g-162.80g=2.2g
180sʱ���ɶ�����̼������Ϊ��15g+50g+20g+20g+20g+20g+20g+20g-182.80g=2.2g
210sʱ���ɶ�����̼������Ϊ��15g+50g+20g+20g+20g+20g+20g+20g+20g-252.00g=-37g
240sʱ���ɶ�����̼������Ϊ��15g+50g+20g+20g+20g+20g+20g+20g+20g+20g-222.80g=2.2g
�ɼ������ݿ�֪����120sʱ��Ӧֹͣ�����ɵ����屣����2.2g���䣬����210sʱ���ݲ��������������岻����Ϊ��ֵ��ҲӦ�ü������������Ϊ2.2g��
�ʴ�Ϊ��210��
ͼ�����£�

��2��Ҫ������ɶ�����̼����������ͨ���ⶨ������̼�������Ȼ���ٸ����ܶ����������Ҳ����������������Һ���ն�����̼���壬ͨ������������Һ�����ı仯������������̼��������
�ʴ�Ϊ��ͨ������������̼�������������Ķ�����̼���ܶȣ������õ�������̼��������
����������������Һ���ն�����̼���壬ͨ���������������Һ�����仯�õ�������̼������������
��3���������Ϸ�����֪��Ʒ�������ַ�Ӧ�����ɵĶ�����̼��������Ϊ2.2g��
����Ʒ��̼��ص�����Ϊx
K2CO3+2HCl=2KCl+H2O+CO2��
138 44
x 2.2g
| 138 |
| 44 |
| x |
| 2.2g |
x=6.9g
����Ʒ��̼��ص�����Ϊ6.9g��
��4������Ʒ�������ַ�Ӧ����ˣ���Һ��ֻ�����Ȼ��أ�
���Ȼ��ص�����Ϊ14.9g
���Ԫ�ص�����=14.9g��
| 39 |
| 39+35.5 |
����Ʒ�м�Ԫ�ص���������=
| 7.8g |
| 15g |
����Ʒ�м�Ԫ�ص�����������52%��
����������ͨ���ⶨ��������Ч�ɷֵ������������������������Ƿ������ݵ���������Ҫ�������Ч���ݽ��л�ѧ����ʽ�ļ����Ԫ�����������ļ��㣮

��ϰ��ϵ�д�
 ��ѧ��ʦ����ϵ�д�
��ѧ��ʦ����ϵ�д�
�����Ŀ
ij��ѧ��ȤС���ڻ��ʳ����з���ij�ּط���Ʒ����ɷ�Ϊ̼��ء��Ȼ��ؼ�����������Ԫ�ص����������ʣ�Ϊ�ⶨ�üط���Ʒ��̼��������ͼ�Ԫ�ص�����������С��������ͬѧ����������ʵ�飺
�ٳ�ȡ15g�ط���Ʒ��������ƿ������Ϊ50g ���У�ͬʱ��װ��ҩƷ����ƿ���ڵ�����ƽ������������ͼ��ʾ��

��ֱ������ƽ�ϵ���ƿ�м���20gϡ���ᣬ����ÿ��30������ƿ�м���20gϡ���ᣬ��������ǰ��ƽ�ϵ�ʾ����¼����
| ʱ��/s | 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 |
| ʾ��/g | 84.45 | 103.90 | 123.35 | 142.80 | 162.80 | 182.80 | 252.00 | 222.80 |
��ȡ��Һ������������������õ����������������ᾧˮ��14.9g
���⣺
��1���ڢڲ�ʵ���м�¼�����ݲ���������ʱ��Ϊ______������ݣ�������Ӧ��ȥ��������ݱ�������ͼ��ʾ����ϵ�л������������ͼ��

��2��ͬѧ����Ϊ�ⶨ������̼������ʵ�鷽����������______��
��3������ʵ���֪��Ʒ��̼�������Ϊ���٣�
��4������Ʒ�м�Ԫ����������Ϊ���٣�
��2010?���ݶ�ģ��ij��ѧ��ȤС���ڻ��ʳ����з���ij�ּط���Ʒ����ɷ�Ϊ̼��ء��Ȼ��ؼ�����������Ԫ�ص����������ʣ�Ϊ�ⶨ�üط���Ʒ��̼��������ͼ�Ԫ�ص�����������С��������ͬѧ����������ʵ�飺
�ٳ�ȡ15g�ط���Ʒ��������ƿ������Ϊ50g ���У�ͬʱ��װ��ҩƷ����ƿ���ڵ�����ƽ������������ͼ��ʾ��

��ֱ������ƽ�ϵ���ƿ�м���20gϡ���ᣬ����ÿ��30������ƿ�м���20gϡ���ᣬ��������ǰ��ƽ�ϵ�ʾ����¼����
�۽���ƿ�еĻ������ˣ�
��ȡ��Һ������������������õ����������������ᾧˮ��14.9g
���⣺
��1���ڢڲ�ʵ���м�¼�����ݲ���������ʱ��Ϊ______������ݣ�������Ӧ��ȥ��������ݱ�������ͼ��ʾ����ϵ�л������������ͼ��

��2��ͬѧ����Ϊ�ⶨ������̼������ʵ�鷽����������______��
��3������ʵ���֪��Ʒ��̼�������Ϊ���٣�
��4������Ʒ�м�Ԫ����������Ϊ���٣�
�ٳ�ȡ15g�ط���Ʒ��������ƿ������Ϊ50g ���У�ͬʱ��װ��ҩƷ����ƿ���ڵ�����ƽ������������ͼ��ʾ��

��ֱ������ƽ�ϵ���ƿ�м���20gϡ���ᣬ����ÿ��30������ƿ�м���20gϡ���ᣬ��������ǰ��ƽ�ϵ�ʾ����¼����
| ʱ��/s | 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 |
| ʾ��/g | 84.45 | 103.90 | 123.35 | 142.80 | 162.80 | 182.80 | 252.00 | 222.80 |
��ȡ��Һ������������������õ����������������ᾧˮ��14.9g
���⣺
��1���ڢڲ�ʵ���м�¼�����ݲ���������ʱ��Ϊ______������ݣ�������Ӧ��ȥ��������ݱ�������ͼ��ʾ����ϵ�л������������ͼ��

��2��ͬѧ����Ϊ�ⶨ������̼������ʵ�鷽����������______��
��3������ʵ���֪��Ʒ��̼�������Ϊ���٣�
��4������Ʒ�м�Ԫ����������Ϊ���٣�