��Ŀ����
ˮ������֮Դ��Ҳ�dz�����������Һ���ܼ�����1�����ݳ�����ѧ֪ʶ����֤��ˮ����H��O��Ԫ����ɵĻ�ѧʵ����
��2��ij��ѧ�о���ѧϰС����Ͼ������ˮ�ʵ�״��������صĵ����о���
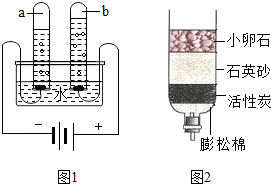
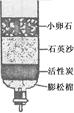
��ȡˮ�������ú���ˣ����˲��������õ��IJ���������©����
����Ҫ���������ˮ����Ӳˮ������ˮ�����õ�������
����Ҫ�ⶨ����ˮ�����ȣ���ѡ��
A����ɫʯ����Һ B����ɫ��̪��Һ C��pH��ֽ
�ܹ�����Աÿ�����С���ں��д�������������������Ʒ��������ӦͶ������
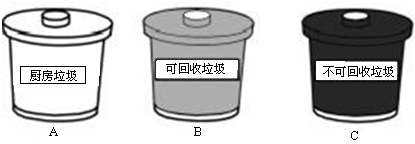
������Ϊ������Ϊ������������ˮ���½�����
A���ں�����ֲ������ľ B������������ˮ�������� C��ijЩ�οͽ��������ֶ������
��3��Ϊ������50g 5%���Ȼ�����Һ����������²������ټ��㣻����������ƽ����
��������1���ع�ѧϰ���ݣ��ҳ���֤��ˮ����H��O��Ԫ����ɵĻ�ѧʵ�飬��д����Ӧ�Ļ�ѧ����ʽ��
��2���ٸ��ݹ��˵�װ�ã��жϹ�������Ҫ��������������ȱ������
�ڸ��ݶ�ˮ��ѧϰ��˵������Ӳˮ������ˮ��ʹ�õ��Լ���
�۸���ָʾ������ֽ�IJ�ͬ��ѡ��ⶨ����ˮ��������Ӧ����ѡ��
���ж�������Ʒ���������Ƿ�ɻ��գ�ѡ����Ӧ�ŵ�λ�ã�
�ݷ�����Ϊ��ˮ��Ӱ�죬ѡ����������ˮ���½�����Ϊ��
��3��������Ҫ������Һ�������������������������ʵ�����������ʽ��������ʡ��ܼ����ļ��㣬������ˮ�����ѡ����ʵ���Ͳ��
��2���ٸ��ݹ��˵�װ�ã��жϹ�������Ҫ��������������ȱ������
�ڸ��ݶ�ˮ��ѧϰ��˵������Ӳˮ������ˮ��ʹ�õ��Լ���
�۸���ָʾ������ֽ�IJ�ͬ��ѡ��ⶨ����ˮ��������Ӧ����ѡ��
���ж�������Ʒ���������Ƿ�ɻ��գ�ѡ����Ӧ�ŵ�λ�ã�
�ݷ�����Ϊ��ˮ��Ӱ�죬ѡ����������ˮ���½�����Ϊ��
��3��������Ҫ������Һ�������������������������ʵ�����������ʽ��������ʡ��ܼ����ļ��㣬������ˮ�����ѡ����ʵ���Ͳ��
����⣺��1��ѧϰ����ͨ�����ˮ��ʵ��˵��ˮ����H��O��Ԫ����ɵģ���Ӧ�Ļ�ѧ����ʽΪ2H2O
2H2��+O2����
��2���ٹ��˲�����Ҫ����Ҫ������©�������������ձ���
������Ӳˮ��ʹ����ˮ����ĭ�������٣�����ˮ����ĭ�Ϸḻ��ͨ��ʹ�÷���ˮ����Ӳˮ����ˮ��
�۷�̪��ʯ��ֻ�ܼ�����Һ������Զ����ܲⶨ��Һ���ȣ�pH��ֽ���Dzⶨ��Һ���ȵ����ģ�
��������Ʒ���������ɻ������ã�Ӧ����BͰ�ɻ�������Ͱ�ڣ�
��A���ں�����ֲ������ľ������Ӱ��ˮ�ʣ�����ȷ��
B������������ˮ����������ʹˮ�ʶ��ʴ���
C��ijЩ�οͽ��������ֶ�����У��������ˮ����Ⱦ���ʴ���
��3������50g 5%���Ȼ�����Һ����Ҫ�Ȼ��Ƶ�����=50g��5%=2.5g����Ҫˮ������=50g-2.5g=47.5g��47.5mL�������Ҫѡ��50mL����Ͳ��
�ʴ�Ϊ��
��1�����ˮ��2H2O
2H2��+O2����
��2�����ձ����ڷ���ˮ����C����B����A��
��3��2.5��50mL��47.5��
| ||
��2���ٹ��˲�����Ҫ����Ҫ������©�������������ձ���
������Ӳˮ��ʹ����ˮ����ĭ�������٣�����ˮ����ĭ�Ϸḻ��ͨ��ʹ�÷���ˮ����Ӳˮ����ˮ��
�۷�̪��ʯ��ֻ�ܼ�����Һ������Զ����ܲⶨ��Һ���ȣ�pH��ֽ���Dzⶨ��Һ���ȵ����ģ�
��������Ʒ���������ɻ������ã�Ӧ����BͰ�ɻ�������Ͱ�ڣ�
��A���ں�����ֲ������ľ������Ӱ��ˮ�ʣ�����ȷ��
B������������ˮ����������ʹˮ�ʶ��ʴ���
C��ijЩ�οͽ��������ֶ�����У��������ˮ����Ⱦ���ʴ���
��3������50g 5%���Ȼ�����Һ����Ҫ�Ȼ��Ƶ�����=50g��5%=2.5g����Ҫˮ������=50g-2.5g=47.5g��47.5mL�������Ҫѡ��50mL����Ͳ��
�ʴ�Ϊ��
��1�����ˮ��2H2O
| ||
��2�����ձ����ڷ���ˮ����C����B����A��
��3��2.5��50mL��47.5��
������������к�ǿ���ۺ��ԣ�ֻ��ͨ�����϶���ѧϰ֪ʶ���й��ɡ������������ڴ�����������ʱ������Ӧ�֣�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��2013?Ϋ����ģ��ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ���ش��������⣺
��2013?Ϋ����ģ��ˮ������֮Դ��Ҳ������������Դ��������ѧ���Ļ�ѧ֪ʶ���ش��������⣺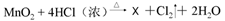 ��
��X�Ļ�ѧʽΪ
���˷�Ӧ��������������� ���ѧʽ�����˷�Ӧ�к�������̬��Ԫ�ص�������
���ѧʽ����
��
��X�Ļ�ѧʽΪ
���˷�Ӧ��������������� ���ѧʽ�����˷�Ӧ�к�������̬��Ԫ�ص�������
���ѧʽ����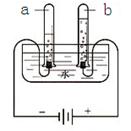
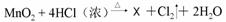 �ݡ�84������Һ����Ч�ɷ�NaClO�е���Ԫ�صĻ��ϼ�Ϊ (11) ��Cl2��������ˮ����������ʵ�����Ʒ�Ϊ �� ��X�Ļ�ѧʽΪ (12) ���˷�Ӧ��������������� (13) ���ѧʽ�����˷�Ӧ�к�������̬��Ԫ�ص������� ��14�� ���ѧʽ����
�ݡ�84������Һ����Ч�ɷ�NaClO�е���Ԫ�صĻ��ϼ�Ϊ (11) ��Cl2��������ˮ����������ʵ�����Ʒ�Ϊ �� ��X�Ļ�ѧʽΪ (12) ���˷�Ӧ��������������� (13) ���ѧʽ�����˷�Ӧ�к�������̬��Ԫ�ص������� ��14�� ���ѧʽ����