��Ŀ����
��1���ⶨ�������о�
������������Ϊ0.07%��NaNO2����Һ���ֱ�ȡ0��2��4��6��8��10 mL����Һ��6֧�Թܣ�������ˮ���������Ϊl0mL���ֱ����Լ0.30gM��δ��M�ǰ��������ᣩ�����Ƴ���ɫ��dz��ͬ�ı�ɫ�ף��ⶨʱȡ10 mLˮ��������Լ0.30gM��ĩ�������ɫ�ױȽϣ��ó���ɫ��ͬ��ɫ����������Һ�ĺ��������ٰ�ϡ�ͱ��������ˮ����NaNO2������������ˮ��ϡ��Һ���ܶȾ���l g?cm-3���㣩��
��������������Ϊ0.07%��NaNO2��Һl000 mL������NaNO2��������
�������������ⶨijˮ�������뺬2 mL����Һ��ɫ����ɫ��ͬ����ˮ����NaNO2����������Ϊ
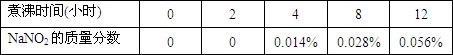
��2���о�ijˮ���ڲ�ͬ���ʱ���NaNO2�����ı仯����һˮ������ͼװ����У��ⶨ��ͬ���ʱ��NaNO2���������������Ϊ��
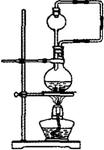
����ȤС����Ƶ���ͼʵ��װ���õ�����������װ�ã���������
�ڸ����������ݣ��ɼ���ˮ�������ʱ�ʵ��ӳ�NaNO2��������������
��3��Ѱ���ܽ���ˮ��NaNO2���������ʣ��о�������Ⱦ����Ч����
�������Ͽ�֪����������˫��ˮ�ɽ���ˮ��NaNO2�����������������ʵ��֤�����������Ƿ��н���ˮ��NaNO2�������������ã���д����ʵ�鱨�棺
| ʵ�鲽�� | ���ܵ�ʵ������ | ���ݿ��ܵ�ʵ������ó��Ľ��� |
��1�����ñ���Һ���Ʋ�ͬ��������������NaNO2��Һ10mL��Ȼ��ֱ����Լ0.30g�����������ĩ���Ƚ���ɫ��dz���Ӷ���֪��ҺNaNO2�ĺ�����
�ٸ������������������㹫ʽ���ɵ�����������
�������2mL��NaNO2��Һ���������������ٳ���10mL��Һ������������ô�ˮ����NaNO2������������
��3�����ʵ��ͨ���Ӵ����˫��ˮ�ķ������ɽ���ˮ��NaNO2����������������ʵ�鲽�衢����ͽ��ۣ�
�ڣ�2 mL��l g?cm-3��0.07%���£�10mL��l g?cm-3��=0.014%
��2���������ʵ��װ�ú����ݿ�֪��������������Ŀ���DZ����ܼ����������䣬��NaNO2������������������
��3�����ݣ�1���ⶨ�������ʵ�飬Ԥ������ͽ��ۣ�
| ʵ�鲽�� | ���ܵ�ʵ������ | ���ܵĽ��� |
| ȡ10 mL NaNO2����Һ���Թܣ��������δ����˫��ˮ�����ټ���0.3gM��ĩ�������ɫ�ױȽ� | ��Һ��ɫ��10 mLNaNO2��ɫ����ɫdz | ���������н���NaNO2������ |

 18���������ƣ�NaNO2����һ���°����ʣ�ij��ȤС������ͼ��ʾ��װ�ò��ijˮ��������ͬ���ʱ���NaNO2�����ı仯���±����������й�˵����ȷ���ǣ������� 18���������ƣ�NaNO2����һ���°����ʣ�ij��ȤС������ͼ��ʾ��װ�ò��ijˮ��������ͬ���ʱ���NaNO2�����ı仯���±����������й�˵����ȷ���ǣ�������
|
 18���������ƣ�NaNO2����һ���°����ʣ�ij��ȤС������ͼ��ʾ��װ�ò� ��ijˮ��������ͬ���ʱ���NaNO2�����ı仯���±������������й�˵������ȷ���ǣ������� 18���������ƣ�NaNO2����һ���°����ʣ�ij��ȤС������ͼ��ʾ��װ�ò� ��ijˮ��������ͬ���ʱ���NaNO2�����ı仯���±������������й�˵������ȷ���ǣ�������
|
NaNO2��—���°����ʡ�ij��ȤС��Ϊ�о�ˮ��NaNO2�ĺ�����������Ⱦ�ķ���������������Ŀ�ӱ�ɫ��ʵ��(�Ƚ���Һ��ɫ��dz�Բⶨ����Ũ�ȵķ���)���������ʵ�飬����գ�
(1)�ⶨ�������о�
������������Ϊ0.07����NaNO2����Һ���ֱ�ȡ0��2��4��6��8��10 mL����Һ��6֧�Թܣ�������ˮ���������Ϊl0mL���ֱ����Լ0.30gM��δ(M�ǰ���������)�����Ƴ���ɫ��dz��ͬ�ı�ɫ�ס��ⶨʱȡ10 mLˮ��������Լ0.30gM��ĩ�������ɫ�ױȽϣ��ó���ɫ��ͬ��ɫ����������Һ�ĺ��������ٰ�ϡ�ͱ��������ˮ����NaNO2����������(ˮ��ϡ��Һ���ܶȾ���l g·cm-3����)��
��������������Ϊ0.07����NaNO2��Һl000 mL������NaNO2��������_________g��
�������������ⶨijˮ�������뺬2 mL����Һ��ɫ����ɫ��ͬ����ˮ����NaNO2����������Ϊ___________��
 (2)�о�ijˮ���ڲ�ͬ���ʱ���NaNO2�����ı仯
(2)�о�ijˮ���ڲ�ͬ���ʱ���NaNO2�����ı仯
����һˮ������ͼװ����У��ⶨ��ͬ���ʱ��NaNO2���������������Ϊ��
| ���ʱ��(Сʱ) | 0 | 2 | 4 | 8 | 12 |
| NaNO2���������� | 0 | 0 | 0.014% | 0.028% | 0.056% |
����ȤС����Ƶ���ͼʵ��װ���õ�����������װ�ã���������_______________��
�ڸ����������ݣ��ɼ���ˮ�������ʱ�ʵ��ӳ�NaNO2��������������_________��
{3)Ѱ���ܽ���ˮ��NaNO2���������ʣ��о�������Ⱦ����Ч����
�������Ͽ�֪����������˫��ˮ�ɽ���ˮ��NaNO2�����������������ʵ��֤�����������Ƿ��н���ˮ��NaNO2�������������ã���д����ʵ�鱨�棺
| ʵ�鲽�� | ���ܵ�ʵ������ | ���ݿ��ܵ�ʵ������ó��Ľ��� |
NaNO2��—���°����ʡ�ij��ȤС��Ϊ�о�ˮ��NaNO2�ĺ�����������Ⱦ�ķ�������������Ŀ�ӱ�ɫ��ʵ��(�Ƚ���Һ��ɫ��dz�Բⶨ����Ũ�ȵķ���)���������ʵ�鲢��գ�
(1)�ⶨ�������о�
������������Ϊ0.07����NaNO2����Һ���ֱ�ȡ0��2��4��6��8��10 mL����Һ��6֧�Թܣ�������ˮ���������Ϊl0mL���ֱ����Լ0.30gM��δ(M�ǰ���������)�����Ƴ���ɫ��dz��ͬ�ı�ɫ�ס��ⶨʱȡ10 mLˮ��������Լ0.30gM��ĩ�������ɫ�ױȽϣ��ó���ɫ��ͬ��ɫ����������Һ�ĺ��������ٰ�ϡ�ͱ��������ˮ����NaNO2����������(ˮ��ϡ��Һ���ܶȾ���l g·cm-3����)��
��������������Ϊ0.07����N aNO2��Һl000 mL������NaNO2��������________g��
aNO2��Һl000 mL������NaNO2��������________g��
�������������ⶨijˮ�������뺬2 mL����Һ��ɫ����ɫ��ͬ����ˮ����NaNO2����������Ϊ______��
(2)�о�ijˮ���ڲ�ͬ���ʱ���NaNO2�����ı仯
����һˮ������ͼװ����У��ⶨ��ͬ���ʱ��NaNO2���������������Ϊ��
| ���ʱ��(Сʱ) | 0 | 2 | 4 | 8 | 12 |
| NaNO2���������� | 0 | 0 | 0.014% | 0.028% | 0.056% |
����ȤС����Ƶ���ͼʵ��װ���õ�����������װ�ã���������_______________��
�ڸ����������ݣ��ɼ���ˮ�������ʱ�ʵ��ӳ�NaNO2��������������____��
(3)Ѱ���ܽ���ˮ��NaNO2�� �������ʣ��о�������Ⱦ����Ч����
�������ʣ��о�������Ⱦ����Ч����
�������Ͽ�֪����������˫��ˮ�ɽ���ˮ��NaNO2�����������������ʵ��֤�����������Ƿ��н���ˮ��NaNO2�������������ã���д����ʵ�鱨�棺
| ʵ�鲽 | ���ܵ�ʵ������ | ���ܵĽ��� |

 ��
��

