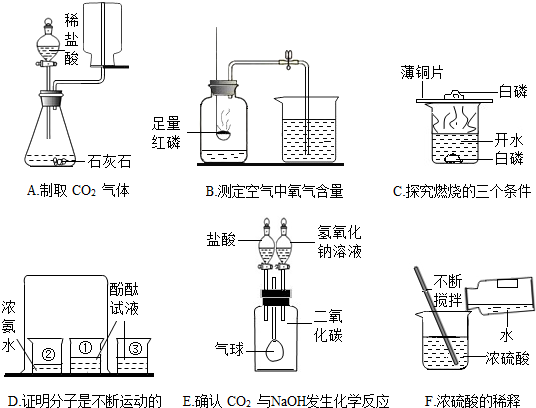
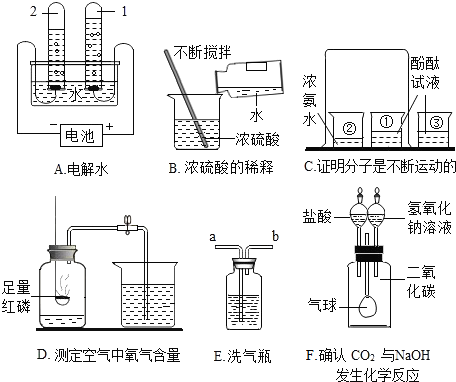
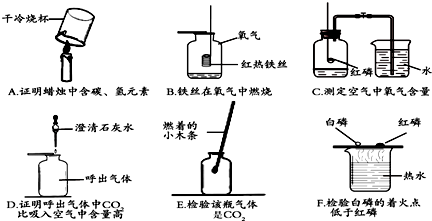
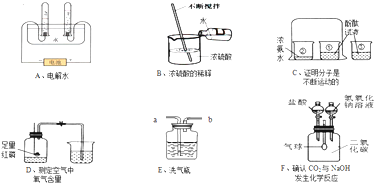
��Ŀ����
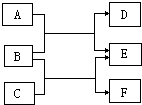 A��F�dz��л�ѧ�еij������ʣ���������ͼ��ʾ��ת����ϵ���ش��������⣺
A��F�dz��л�ѧ�еij������ʣ���������ͼ��ʾ��ת����ϵ���ش��������⣺
��1����AΪ��̬���ʣ�CΪ��̬���ʣ�EΪ��ɫ��̬���ʣ���F�Ļ�ѧʽΪ________��A��B��Ӧ�Ļ�ѧ����ʽΪ________��
��2����BΪ���ʡ�DΪ���塢FΪ���塢E��Һ��dz��ɫ����A��������������________����ʡ��ᡢ��Σ���B��C�ķ�Ӧ������________��
��3����A��F��Ϊ�����C�ǵ�������ЧӦ����Ҫ���ʣ�EΪ��ɫ��������A��B��Ӧ�Ļ�����Ӧ������________��Ӧ��C��D��Ӧ�ķ���ʽΪ________��
�⣺��1��EΪ��ɫ��̬���ʣ�˵��EΪͭ��AΪ��̬���ʣ�CΪ��̬���ʣ�������B��Ӧ����ͭ����BΪ����ͭ��A��CΪ���л�ԭ�Ե����ʣ�����AΪ̼��CΪ��������ô����������ͭ��Ӧ��������ͭ����������ˮ������FΪˮ��
�ʱ����Ϊ��H2O��C+2CuO 2Cu+CO2����
2Cu+CO2����
��2��E��Һ��dz��ɫ��˵��E�к��������ӣ��ʸ÷�Ӧ�����μӣ�DΪ���壬˵��A��B�ķ�ӦΪ����������ķ�Ӧ��BΪ���ʣ���A�����ᣬ��+C������F+��������Һ��˵���˷�ӦΪ����������Һ�ķ�Ӧ���ʿ�����ʪ��ұ����жϽ�����Ե�ǿ�������Ա����Ϊ���ᣬʪ��ұ����жϽ������˳��ȣ�
��3��C�ǵ�������ЧӦ����Ҫ���ʣ�EΪ��ɫ������˵��C�Ƕ�����̼��EΪ̼��ƣ���BΪ�������ƣ��������ƿ��������̼���η������ֽⷴӦ����̼��Ƴ�������A������̼���ƣ���ô���ɵ�DΪ�������ƣ����Ա����Ϊ�����ֽ⣬CO2+2NaOH=Na2CO3+H2O�ȣ�
����������Ϊ��ͼʽ�����ƶ��⣬��ɴ�����Ŀ���ؼ���������ͻ�ƿڣ����������Ĺؼ����Լ����ʵ����ʺ�����֮��ķ�Ӧ�������жϣ�
��1��EΪ��ɫ��̬���ʣ�˵��EΪͭ��
��2��E��Һ��dz��ɫ��˵��E�к��������ӣ��ʸ÷�Ӧ�����μӣ�
��3��C�ǵ�������ЧӦ����Ҫ���ʣ�EΪ��ɫ������˵��C�Ƕ�����̼��EΪ̼��ƣ�
����������Ϊ��ͼʽ�����ƶ��⣬��ɴ�����Ŀ���ؼ���������ͻ�ƿڣ�ֱ�ӵó����ۣ�Ȼ������˳���������������м��ƣ���һ�����������ۣ�
�ʱ����Ϊ��H2O��C+2CuO
 2Cu+CO2����
2Cu+CO2������2��E��Һ��dz��ɫ��˵��E�к��������ӣ��ʸ÷�Ӧ�����μӣ�DΪ���壬˵��A��B�ķ�ӦΪ����������ķ�Ӧ��BΪ���ʣ���A�����ᣬ��+C������F+��������Һ��˵���˷�ӦΪ����������Һ�ķ�Ӧ���ʿ�����ʪ��ұ����жϽ�����Ե�ǿ�������Ա����Ϊ���ᣬʪ��ұ����жϽ������˳��ȣ�
��3��C�ǵ�������ЧӦ����Ҫ���ʣ�EΪ��ɫ������˵��C�Ƕ�����̼��EΪ̼��ƣ���BΪ�������ƣ��������ƿ��������̼���η������ֽⷴӦ����̼��Ƴ�������A������̼���ƣ���ô���ɵ�DΪ�������ƣ����Ա����Ϊ�����ֽ⣬CO2+2NaOH=Na2CO3+H2O�ȣ�
����������Ϊ��ͼʽ�����ƶ��⣬��ɴ�����Ŀ���ؼ���������ͻ�ƿڣ����������Ĺؼ����Լ����ʵ����ʺ�����֮��ķ�Ӧ�������жϣ�
��1��EΪ��ɫ��̬���ʣ�˵��EΪͭ��
��2��E��Һ��dz��ɫ��˵��E�к��������ӣ��ʸ÷�Ӧ�����μӣ�
��3��C�ǵ�������ЧӦ����Ҫ���ʣ�EΪ��ɫ������˵��C�Ƕ�����̼��EΪ̼��ƣ�
����������Ϊ��ͼʽ�����ƶ��⣬��ɴ�����Ŀ���ؼ���������ͻ�ƿڣ�ֱ�ӵó����ۣ�Ȼ������˳���������������м��ƣ���һ�����������ۣ�

��ϰ��ϵ�д�
�����Ŀ
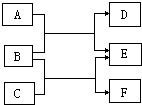 A��F�dz��л�ѧ�еij������ʣ���������ͼ��ʾ��ת����ϵ���ش��������⣺
A��F�dz��л�ѧ�еij������ʣ���������ͼ��ʾ��ת����ϵ���ش��������⣺