��Ŀ����
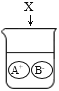
ʵ�����мס�����ƿ���õ��������ƹ��壬ijѧϰС��Ϊ���о���������������������ʵ�飺�����ӳ�ʾ����λΪg��

��1��������ɫʯ����Һ����ҺΪ��ɫ��˵����Ӧ����Һ��_____�ԡ�
��2������ʵ������Ķ�����̼��������Ϊ_____g��
��3�������ƿ������Ʒ��̼���Ƶ���������_____��
��4��ijͬѧ��ȡ10 g��ƿ�еĹ�����Ʒ����100 g 15%��ϡ���ᰴͬ����������ʵ�飬����Ϊ���ܹ�����Ʒ���ʳ̶���Σ�ϡ������������Ҫʹ��ʯ����Һ�������˵�����������жϵ�ԭ����Na2CO3��H2SO4=Na2SO4��H2O��CO2����ÿ106��������̼������ȫ��Ӧ��98�ݵ�H2SO4��100 g15%��ϡ������H2SO4������Ϊ_____g������_____��
 Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�Ԫ�����ڱ���ѧϰ���о���ѧ����Ҫ���ߡ�������±���Ԫ�����ڱ��IJ������ݣ����ش��й����⣺
���� �� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 |
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
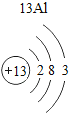
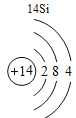
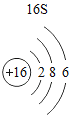
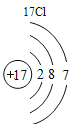
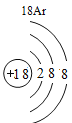
(1)��д��������Ԫ�ص�һ����Ϣ_________________________________��
(2)����3�ź�11��Ԫ��ԭ�ӵ�������������ͬ������__________����õ�����ʧȥ�������ӡ���Ԫ�غͷ�Ԫ����ɻ�����Ļ�ѧʽΪ___________�����ɸ����ʵ�����___________������ӡ�ԭ�ӡ������ӡ�����
(3)��Ԫ�����ڱ��У�ͬһ�壨���У���Ԫ�ؾ������ƵĻ�ѧ���ʡ������и���Ԫ�ؾ������ƻ�ѧ���ʵ���____________������ĸ��ţ���
a.C��Ne
b.Be��Mg
C.Al��Si
d.S��O
(4)��Ԫ�غ���Ԫ���γɵĻ������ˮ��Һ����ᣨHF���������ڲ�����̣�����Ҫԭ����������벣������Ҫ�ɷֶ������裨SiO2��������Ӧ�������ķ��������壨SiF4����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ____________________________��
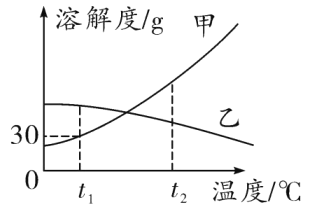
��ͬѧΪ�������������ƺ�����������Һ���������ͼ��ʾ������ʵ�鷽����

��1�������ܴﵽʵ��Ŀ�ĵ���_____��
��2����C��ʵ���з����Ļ�ѧ����ʽΪ_____��
��3��ʵ�������ͬѧ��A��C����֧�Թ��е���Һ����ͬһ�����У�������Һ����ǣ�������˵����_____���ɣ��ѧʽ���������̪����Һ�ʺ�ɫ��
��4�������̪����Һ�ʺ�ɫ��˵����Һ�ʼ��ԡ�ʹ��Һ�ʼ��Ե�������ʲô�أ���ͬѧ��һ���Ʋ⣺��Һ�гʼ��Ե����ʿ�����̼���ơ��������ƺ�NaOH���������е�һ�֣����������������������ɵĻ���
��5��Ϊ����֤�Ʋ⣬��ͬѧ�������ϣ���Ϥ�Ȼ�����Һ�����ԣ����������ʵ�������֤�������Ҫ��������пհף�
ʵ������ | Ԥ������ | ���� |
ȡ���������е��ϲ���Һ���Թ��У�����������Ȼ�����Һ������ | �����а�ɫ��������Һ����ɫ | ������̼���� |
�����������ɣ���Һ�ʺ�ɫ | �������������� | |
�����а�ɫ��������Һ�ʺ�ɫ | ___________________ |
��6����ͬѧ��Ϊ��ͬѧ�ڢڲ���֤�Ľ��۲����ܣ�ԭ����_____��


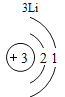
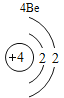
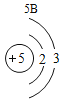
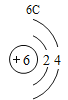
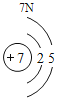
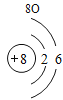
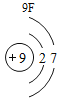
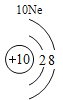
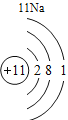
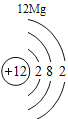
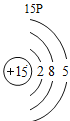
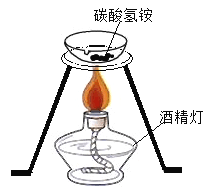 ֤��NH4HCO3���ȷֽ�. B.
֤��NH4HCO3���ȷֽ�. B. ֤������ȼ�����ɶ�����̼��ˮ
֤������ȼ�����ɶ�����̼��ˮ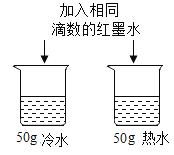 ̽���¶ȶԷ����˶�������Ӱ�� D.
̽���¶ȶԷ����˶�������Ӱ�� D. ֤��������̼�ܶȱȿ�����
֤��������̼�ܶȱȿ�����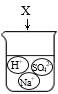 B.
B.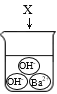 C.
C.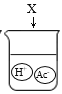 D.
D.