��Ŀ����
����Ŀ��ijƷ��άC����Ƭ��ǩ��ͼ1��ʾ��Ϊ�о������ʲ��ⶨ����NaHCO3�������������̽�����̡�
��1��άC����Ƭ�������л�����г�ζ���ϡ������ᡢ��
��2����άC����ƬͶ��ˮ�У��۲쵽�д������ݲ�������Ҫ����Ϊ�����ᣨH3C6H5O7����NaHCO3������Ӧ�������Ƹ÷�Ӧ��3NaHCO3+H3C6H5O7= Na3C6H5O7+3 H2O+��
��3��Ϊ�ⶨNaHCO3���������ͼ2װ�ã������������������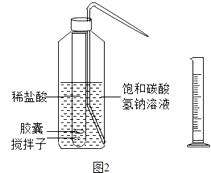
ʵ�鲽�裺��ȡ��Ʒm gװ������У���С�Թ��з���С�ʹ��������ӣ��ټ�������ϡ���ᣬ�ٰѽ��ҷŵ��Թ��У���500 mLϴƿ��װ��һ�����ı���̼��������Һ����С�Թ�С�ķ���ϴƿ����ͼ2�����Ǻ�ϴƿ�ĸ��ӣ����ڴ����������ϣ������ܽ⣬����Ƭ���������ã���Ӧ�ӽ���ɣ���������ֱ��ϴƿ����Һ������������Ͳ��ȡϴƿ��������Һ�����ΪV mL��ʹ�ý��ҵ�Ŀ���� �� �����ӵ������� �� ����̼�����ƴ���ˮ�����Է�ֹ����֪���¶���CO2�ܶ����� g/mL����άC����Ƭ��NaHCO3��������
��4�����в������ܵ��²ⶨ���ƫС������
a��С�Թ������������ϴƿ��
b��ʵ�������������Ͳ����
c������m gάC����Ƭʱ��������������ȷ��������ƽָ��ƫ��������
d��άC����Ƭ��װ�������ʱ��������
��5��ijͬѧ���װ�ã���ͼ3���ⶨNaHCO3������ʵ����Ʒ��ϡ���ᷴӦ�IJ����� �� ���ⶨNaHCO3������Ҫ���������������
a����Ʒ����m g
b����Ӧǰ��Aװ������a g��a1 g
c����Ӧǰ��Bװ������b g��b1 g
d����Ӧǰ��Cװ������c g��c1 g
��ѡ������һ�����ݣ���ʾNaHCO3������ָ����װ�õ�һ��ȱ������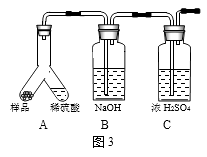
���𰸡�
��1�����Ǻ�ά����C
��2��3CO2
��3����ֹάC����Ƭ��NaHCO3�������ᷴӦ����CO2�ݳ�,ʹ��Ӧ���,CO2�ܽ�,21��v/11m
��4��bd
��5����Y����������б,ʹ����������Ʒһ��,ab,ac,![]() ��
�� ![]() ,���ɵĶ�����̼δ����ȫ�ų���δ�ܱ�NaOH��ȫ����
,���ɵĶ�����̼δ����ȫ�ų���δ�ܱ�NaOH��ȫ����
����������������Ϣ֪����1��άC����Ƭ�������л�����г�ζ���ϡ������ᡢ���Ǻ�ά����C����2����άC����ƬͶ��ˮ�У��۲쵽�д������ݲ�������Ҫ����Ϊ�����ᣨH3C6H5O7����NaHCO3������Ӧ���÷�Ӧ��3NaHCO3+H3C6H5O7= Na3C6H5O7+3H2O+ 3CO2 ������3���ⶨNaHCO3������ʹ�ý��ҵ�Ŀ����. ��ֹάC����Ƭ��NaHCO3�������ᷴӦ����CO2�ݳ��������ӵ�������ʹ��Ӧ��֡�����̼�����ƴ���ˮ�����Է�ֹCO2�ܽ⡣��֪���¶���CO2�ܶ����� g/mL����άC����Ƭ��NaHCO3�����ǡ����ɶ�����̼�����Ǧ�vg. NaHCO3��CO2,, ![]() ��
�� ![]() ,x��21��v/11g. ��4���������ܵ��²ⶨ���ƫС����b��ʵ�������������Ͳ����. d��άC����Ƭ��װ�������ʱ��������. ��5���ⶨNaHCO3������ʵ����Ʒ��ϡ���ᷴӦ�IJ����ǽ�Y����������б��ʹ����������Ʒһ�ˡ����ⶨNaHCO3������Ҫ�����������a����Ʒ����m g��b����Ӧǰ��Aװ������a g��a1 g��a����Ʒ����m g��c����Ӧǰ��Bװ������b g��b1 g����ѡ������һ�����ݣ���ʾNaHCO3������
,x��21��v/11g. ��4���������ܵ��²ⶨ���ƫС����b��ʵ�������������Ͳ����. d��άC����Ƭ��װ�������ʱ��������. ��5���ⶨNaHCO3������ʵ����Ʒ��ϡ���ᷴӦ�IJ����ǽ�Y����������б��ʹ����������Ʒһ�ˡ����ⶨNaHCO3������Ҫ�����������a����Ʒ����m g��b����Ӧǰ��Aװ������a g��a1 g��a����Ʒ����m g��c����Ӧǰ��Bװ������b g��b1 g����ѡ������һ�����ݣ���ʾNaHCO3������ ![]() ��
�� ![]() �����ɵĶ�����̼δ����ȫ�ų���δ�ܱ�NaOH��ȫ���ա�
�����ɵĶ�����̼δ����ȫ�ų���δ�ܱ�NaOH��ȫ���ա�
���Դ��ǣ����Ǻ�ά����C��3CO2����ֹάC����Ƭ��NaHCO3�������ᷴӦ����CO2�ݳ���ʹ��Ӧ��֡�CO2�ܽ⡢21��v/11m����Y����������б��ʹ����������Ʒһ�ˡ�bd��ac��![]() �����ɵĶ�����̼δ����ȫ�ų���δ�ܱ�NaOH��ȫ���ա�
�����ɵĶ�����̼δ����ȫ�ų���δ�ܱ�NaOH��ȫ���ա�
�����㾫�������ø��ݻ�ѧ��Ӧ����ʽ�ļ������Ŀ�����жϼ��ɵõ��𰸣���Ҫ��֪�����ʼ�������=ϵ������Է�������֮�ȣ�

 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д�