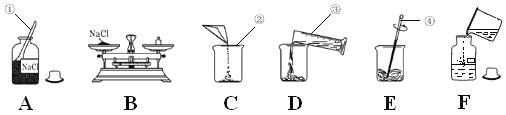
��Ŀ����
����Ŀ������ͼ��ʾװ�ã��ڳ����£������о��������ʵ�ʵ�飮

���ݲ��� | ��ʵ��1���о��������� |
�� | �ձ���ʢ��ʯ��ˮ��ȼ�ճ��з���ľ̿����ȼľ̿��Ѹ�ٽ�ȼ�ճ�����ƿ�У�����ƿ�� |
�� | ��������H2O2 ��Һ |
(1)ʵ��1�У�����H2O2��Һ����ƿ��������_______________________________������H2O2��Һ�۲쵽�ձ��е�������____________________��
(2)����H2O2��Һ�۲쵽ľ̿ȼ�յø����ң��ɴ˵ó�������������_________��ľ̿Ϩ�����ȴһ��ʱ�䣬�ձ��еIJ�����Һ���뼯��ƿ����ʵ���У�����ƿ��ѹǿ�ı仯������__________________��
���𰸡� ľ̿���� ������ ʯ��ˮ����� ��ȼ �ȱ��һ��ʱ����С
��������(1)ʵ��1�У�����H2O2��Һ���ڶ������̵������£���������ֽ�������������������ȼ�ԣ�����ƿ�е�ľ̿����ȼ�գ����������⣻���ɶ�����̼�����ձ��У����������Ʒ�Ӧ����̼��Ƴ�����ˮ���ձ��е�������������ð���������ʯ��ˮ����ǡ�(2)����H2O2��Һ�۲쵽ľ̿ȼ�յø����ң��ɴ˵ó���������������ȼ�ԣ�ľ̿ȼ��ʱ�ų������ȣ������������ͣ�ѹǿ����ľ̿Ϩ�����ȴһ��ʱ�䣬����ƿ������������٣�ѹǿ��С��

����Ŀ��ij��ѧС��ⶨ���������������������
(1)������ͼ�ش����⡣

�ٺ���ȼ�յ����ֱ���ʽΪ_______________________��
�ں���ȼ��ʱ��������___________________________����ȴ�����´�ֹˮ�к۲쵽���������ձ��е�ˮ_______________��
����ȴ�����º��ֹˮ�У��������������ԭ����_______________________________��
��ʵ����ۣ�___________________________________________
(2)��������(Na2S4)�����������ײⶨ���������������������
��Ӧԭ��Ϊ:2Na2S4+O2+2H2O ���� 8S��+4NaOH(��������)��
С���ϣ�������(Na2S4)��������ˮ��Ӧ����������ˮ�Ĺ�����(S)��������ˮ���������ơ�
��ʵ����̡�
��ȡ�����������ƹ�������Թ��У��ټ���������ˮ��Ѹ���������������������Һ�����������صľ��룬��¼����h1(��ͼ1��ʾ)��
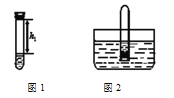
�ڽ����Թܲ���ˮ��(��ͼ2��ʾ)�������������۲쵽_____________���������������Թ�ȡ������ת����������Һ�����������صľ��룬��¼����h2��������h2��h1=______________��
�۰��բٺ͢����ظ�ʵ��2�Ρ�3��ʵ���������±���ʾ��
��1�� | ��2�� | ��3�� | |
h1/cm | 11.0 | 11.4 | 11.6 |
h2/cm | 8.7 | 9.1 | 9.2 |
���ݵ�3��ʵ�����ݣ�����������������������Ϊ________%(�����ȷ�� 0.1%)��