��Ŀ����
��2010?���գ�ij��ȤС����Ҫ10%������������Һ��10%��������Һ�������кͷ�Ӧʵ�飮
��1��������40g��������������Һ������Ҫ�������ƹ��������Ϊ
��2��Ϊ�к���������������Һ����10%��������Һ������Ϊ
��1��������40g��������������Һ������Ҫ�������ƹ��������Ϊ
4
4
g�����±���ѡ������������ƹ�������Ҫ������ACE
ACE
��������ţ�
| ���� | ������ƽ | �ƾ��� | С�ձ� | ������ | Կ�� | ��Ͳ |
| ���� |  |
 |
 |
 |
 |
 |
| ��� | A | B | C | D | E | F |
49
49
g�����㷴Ӧ��������Һ���������������Ƕ��٣�����ȷ��1����������1��������Һ���������������ĸ������Һ��������Һ��������������Һ��������������������ȡ���������ƾ��и�ʴ�ԣ�Ӧ���ڲ��������г������ݴ�ѡ��������
��2�������������������ᷴӦ�Ļ�ѧ����ʽ��ȷ����Ӧ�������������������ʵ�������ϵ�����������Ƶ��������㷴Ӧ��Ҫ����10%��������Һ����������Ӧ���������Ƶ���������ɼ��㣮
��2�������������������ᷴӦ�Ļ�ѧ����ʽ��ȷ����Ӧ�������������������ʵ�������ϵ�����������Ƶ��������㷴Ӧ��Ҫ����10%��������Һ����������Ӧ���������Ƶ���������ɼ��㣮
����⣺��1������40g������������Ϊ10%������������Һ����Ҫ�������Ƶ�����=40g��10%=4g���������Ƶ�ǿ�Ҹ�ʴ��Ҫ���ڳ�����������ʱ�ͷ��ڲ��������ڣ���˳�����������ʱӦѡ��������ƽ���ձ���ҩ������������
��2������10%��������Һ������Ϊx����Ӧ���������Ƶ�����Ϊy
2NaOH+H2SO4�TNa2SO4+2H2O
80 98 142
4g x?10% y
=
x=49g
=
y=7.1g
��Ӧ��������Һ��������������=
��100%=7.98%��8%
�ʴ�Ϊ����1��4��ACE����2��49��8%��
��2������10%��������Һ������Ϊx����Ӧ���������Ƶ�����Ϊy
2NaOH+H2SO4�TNa2SO4+2H2O
80 98 142
4g x?10% y
| 80 |
| 98 |
| 4g |
| x?10% |
| 80 |
| 142 |
| 4g |
| y |
��Ӧ��������Һ��������������=
| 7.1g |
| 40g+49g |
�ʴ�Ϊ����1��4��ACE����2��49��8%��
���������������غ㶨�ɣ�����������Һ��ϡ���ᷴӦ��������Ҳ������������˷�Ӧ��������Һ��Ϊ����������Һ��ϡ����������ͣ�

��ϰ��ϵ�д�
 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
�����Ŀ
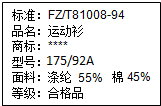 ��2010?���գ���ͼ��ij��װ��ǩ�IJ������ݣ������й���ʶ��ȷ���ǣ�������
��2010?���գ���ͼ��ij��װ��ǩ�IJ������ݣ������й���ʶ��ȷ���ǣ�������