��Ŀ����
����Ŀ���ҹ������Ŷ��״κϳ��˵�ԭ�Ӳ������������������ԭ�Ӵ������ ��ԭ�Ӵ������ںϳ�������ȼ�ϼ״���CH3OH������ʾ��ͼ�� 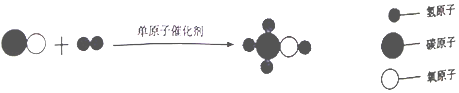
��1��������ͼ����ת������ʾ��ͼ���ж������й�˵���������������ĸ��ţ���
A.ʹ�õ�ԭ�Ӵ��������������IJ���
B.�÷�Ӧǰ��ԭ�ӵ�����û�б仯
C.������״������л���
D.��Ӧ��������ﶼ�ɷ��ӹ��ɣ�
��2��������ͼд����ԭ�Ӵ������ںϳ�������״���Ӧ�Ļ�ѧ����ʽ ��
��3���ϳ�����CO��H2���ڲ�ͬ�����������£����Ժϳɲ�ͬ�����ʣ�������úϳ���Ϊԭ�ϲ����ܵõ���������������ĸ��ţ���
A.����[CO��NH2��2]
B.�����ǣ�C6H12O6��
C.��ȩ��CH2O��
���𰸡�
��1��A
��2��CO+2H2 ![]() CH3OH
CH3OH
��3��A
���������⣺��ͼ����Ϣ��֪��̼��ˮ�ڵ�ԭ�Ӵ��������·�Ӧ�����ɼ״�����Ӧ�Ļ�ѧ����ʽΪ��CO+2H2 ![]() CH3OH����1��A��ʹ�õ�ԭ�Ӵ�������߷�Ӧ���ʣ��������������IJ������ʴ���B����ѧ��Ӧǰ��ԭ�ӵ����ࡢ��Ŀû�б仯������ȷ��C��������״��к���̼Ԫ�أ������л������ȷ��D��CO��H2��CH3OH���ɷ��ӹ��ɣ�����ȷ����2����ͼ����Ϣ��֪��̼��ˮ�ڵ�ԭ�Ӵ��������·�Ӧ�����ɼ״�����Ӧ�Ļ�ѧ����ʽΪ��CO+2H2
CH3OH����1��A��ʹ�õ�ԭ�Ӵ�������߷�Ӧ���ʣ��������������IJ������ʴ���B����ѧ��Ӧǰ��ԭ�ӵ����ࡢ��Ŀû�б仯������ȷ��C��������״��к���̼Ԫ�أ������л������ȷ��D��CO��H2��CH3OH���ɷ��ӹ��ɣ�����ȷ����2����ͼ����Ϣ��֪��̼��ˮ�ڵ�ԭ�Ӵ��������·�Ӧ�����ɼ״�����Ӧ�Ļ�ѧ����ʽΪ��CO+2H2 ![]() CH3OH����3���������غ㶨�ɿ�֪��ѧ��Ӧǰ��Ԫ�ص�����䣬��֪��ӦǰԪ�������ּ�̼��������Ӧ����ֵ�Ԫ�أ��������ز����ܲ������𰸣���1��A����2��CO+2H2
CH3OH����3���������غ㶨�ɿ�֪��ѧ��Ӧǰ��Ԫ�ص�����䣬��֪��ӦǰԪ�������ּ�̼��������Ӧ����ֵ�Ԫ�أ��������ز����ܲ������𰸣���1��A����2��CO+2H2 ![]() CH3OH����3��A��
CH3OH����3��A��
�����㾫����������Ŀ����֪������������д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ�����֪ʶ���Եõ�����Ĵ𰸣���Ҫ����ע�⣺a����ƽ b������ c�����ţ�
