��Ŀ����
��ֽ���ҹ��Ŵ��Ĵ�����֮һ������Ч���ƶ������������ķ�չ��
��1������ũ������Ľո���Ϊ��ֽȼ�ϣ����Լ��ٻ��սոѴ�����______��Ⱦ���ոѵ���Ҫ�ɷ�����ά�ء���C6H10O5��n������ά����C��H��O����Ԫ�ص�������Ϊ______��
��2����ֽ���������NaOH�ļ��Է�ˮ���辭�����������Ժ�����ŷţ�����ˮ�ʼ��Եļ�����______��
��3��ij��ֽ����ˮ�к�NaOH����������Ϊ1.6%�����з�Ʒ����9.8�֣�H2SO4����������Ϊ20%�������Դ����ķ�ˮ�����Ƕ��٣�
�⣺��1�����սոѻ����������������Ⱦ������
������ά�صĻ�ѧʽ��C6H1005��n֪����ά����C��H��O����Ԫ�ص�������=��12��6������1��10������16��5��=36��5��40
�ʴ�Ϊ��������36��5��40��
��2��������Һ�ļ��Գ������ָʾ����PH��ֽ�������÷�̪��Һ���м��飬�����֤����Һ�ʼ��ԣ�
�ʴ�Ϊ��ȡ������ˮ���Թ��У��μӷ�̪��Һ����죮
��3���⣺9.8t��������Һ�к����������Ϊ9.8t��20%=1.96t
��9.8t��������Һ���Է�Ӧ�������Ƶ�����Ϊx
H2SO4 +2NaOH=Na2SO4 +2H2O
98 80
1.96t x
�б���ʽΪ =
=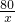
��֮�ã�x=1.6t
��1.6t�������Ƶķ�ˮ������= =100t
=100t
�𣺿��Դ����ķ�ˮ������100t��
��������1����ѧʽ�и�Ԫ�ص�������Ϊ��Ԫ�ص����ԭ��������ԭ�Ӹ�����֮�ȣ�
��2��������Һ�ļ��Գ������ָʾ����pH��ֽ��
��3�����ȸ��ݻ�ѧ����ʽ����������Ƶ��������ڸ��ݷ�ˮ���������Ƶ��������������ˮ��������
������������Ҫ���黯ѧʽ�ĺ��弰���ݻ�ѧ����ʽ���м����������
������ά�صĻ�ѧʽ��C6H1005��n֪����ά����C��H��O����Ԫ�ص�������=��12��6������1��10������16��5��=36��5��40
�ʴ�Ϊ��������36��5��40��
��2��������Һ�ļ��Գ������ָʾ����PH��ֽ�������÷�̪��Һ���м��飬�����֤����Һ�ʼ��ԣ�
�ʴ�Ϊ��ȡ������ˮ���Թ��У��μӷ�̪��Һ����죮
��3���⣺9.8t��������Һ�к����������Ϊ9.8t��20%=1.96t
��9.8t��������Һ���Է�Ӧ�������Ƶ�����Ϊx
H2SO4 +2NaOH=Na2SO4 +2H2O
98 80
1.96t x
�б���ʽΪ
 =
=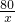
��֮�ã�x=1.6t
��1.6t�������Ƶķ�ˮ������=
 =100t
=100t�𣺿��Դ����ķ�ˮ������100t��
��������1����ѧʽ�и�Ԫ�ص�������Ϊ��Ԫ�ص����ԭ��������ԭ�Ӹ�����֮�ȣ�
��2��������Һ�ļ��Գ������ָʾ����pH��ֽ��
��3�����ȸ��ݻ�ѧ����ʽ����������Ƶ��������ڸ��ݷ�ˮ���������Ƶ��������������ˮ��������
������������Ҫ���黯ѧʽ�ĺ��弰���ݻ�ѧ����ʽ���м����������

��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�
�����Ŀ