��Ŀ����
28���ش��������⣬д���йػ�ѧ����ʽ��
��1����һ���������������������ϡ�����У���ʼʱ���۲쵽��������
��2�������ᱵ��Һ�еμ����ᣬ�۲쵽��������
��1����һ���������������������ϡ�����У���ʼʱ���۲쵽��������
�����ܽ⣬��Һ��Ϊ��ɫ
����Ӧ�Ļ�ѧ����ʽΪ6HCl+Fe2O3�T2FeCl3+3H2O
����2�������ᱵ��Һ�еμ����ᣬ�۲쵽��������
�а�ɫ��������
����Ӧ�Ļ�ѧ����ʽΪH2SO4+Ba��NO3��2�TBaSO4��+2HNO3
������ʵ������Ӧ�ܹ�������ԭ�������ᱵ��Һ�к���Ba2+
��д���ӷ��ţ��������к���SO42-
��д���ӷ��ţ�����������1�������������Ҫ�ɷ����������������������ܺ����ᷴӦ�����Ȼ�����ˮ���Ȼ�����Һ�ǻ�ɫ�ģ�
��2����������Ӻͱ������ܽ�ϳɰ�ɫ�����ᱵ�����ᱵ������ˮ��
��2����������Ӻͱ������ܽ�ϳɰ�ɫ�����ᱵ�����ᱵ������ˮ��
����⣺��1���������Ҫ�ɷ��������������������������������ϡ�����У���ʼʱ���ῴ�������ܽ⣬��Һ����ɫ��ɻ�ɫ����Ӧ�Ļ�ѧ����ʽΪ6HCl+Fe2O3�T2FeCl3+3H2O��
��2�������д�����������ӣ������ᱵ���б����ӣ���������Ӻͱ�������ͬһ��Һ�в����棬�����ɲ�����ˮҲ������������ᱵ��������Ӧ�Ļ�ѧ����ʽΪH2SO4+Ba��NO3��2=BaSO4��+2HNO3��
�ʴ�Ϊ����1�������ܽ⣬��Һ��Ϊ��ɫ��6HCl+Fe2O3�T2FeCl3+3H2O��
��2���а�ɫ�������ɣ�H2SO4+Ba��NO3��2�TBaSO4��+2HNO3�� Ba2+��SO42-��
��2�������д�����������ӣ������ᱵ���б����ӣ���������Ӻͱ�������ͬһ��Һ�в����棬�����ɲ�����ˮҲ������������ᱵ��������Ӧ�Ļ�ѧ����ʽΪH2SO4+Ba��NO3��2=BaSO4��+2HNO3��
�ʴ�Ϊ����1�������ܽ⣬��Һ��Ϊ��ɫ��6HCl+Fe2O3�T2FeCl3+3H2O��
��2���а�ɫ�������ɣ�H2SO4+Ba��NO3��2�TBaSO4��+2HNO3�� Ba2+��SO42-��
������������Ҫ�������ʼ�����Ӧ��������ֽⷴӦ����ʽ����д���������ɷ�Ӧ���ص���ɣ����ڻ���֪ʶ���Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
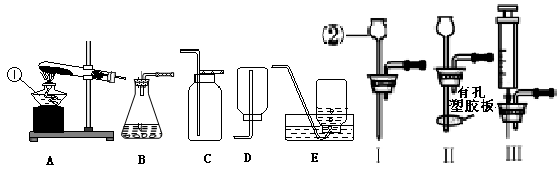
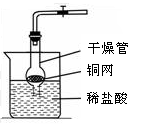
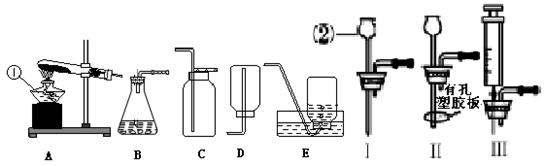
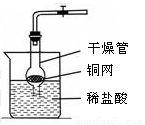
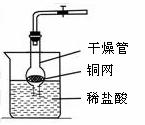
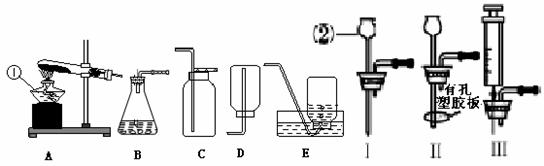 ��д���б�����������ƣ���_____ ______����_______________��
��д���б�����������ƣ���_____ ______����_______________��