��Ŀ����
����������������벻����������1��Ŀǰ�������ߵĽ����� ��
��2�����ڳ�ʪ�Ŀ�����ᷢ����ʴ��֤������һ���μ��˷�Ӧ����Ҫ����ʵ���� ��
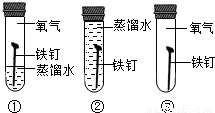
A���٢�B���٢�C���ڢ�D���٢ڢ�
��3��Fe2O3����Ԫ�ص���������Ϊ ��ij����������Ҫ�ɷ��ǣ�Fe2O3���ij�����ʯ������������ÿ����Ҫ����1000t������20%�ij�����ʯ���ó������Ͽɲ���Fe98%�������������� ��
��4��������������ˮ�����������ǿ����Һ������������������Һ����Ӧ����ƫ�����ƣ�NaAlO2����ˮ������������Ϣд��������������������Һ��Ӧ�Ļ�ѧ����ʽ�� ��
��5��������ͭ������п��ϡ����Ļ����Һ�У�������������ۣ�������ʣ�ࣩ��ʹ֮��ַ�Ӧ���ˣ��������ijɷ��� ����Һ�к��е����� ��
���𰸡���������1��Ŀǰ�������������ߵĽ���������
��2��Ҫ��֤��������ʱ������һ���μ��˷�Ӧ����Ҫ��������������ˮ��ͬ�����µķ�Ӧʵ�������ֻ��ˮ��û�������µ�ʵ�飮
��3���������ʵĻ�ѧʽ���Լ���ij��Ԫ�ص�����������ij�����ʵ�������
��4��������Ϣ����������������������Һ����Ӧ����ƫ�����ƣ�NaAlO2����ˮ��д����ѧ����ʽ��
��5����ǽ������˳��K��Ca��Na��Mg��Al��Zn��Fe��Sn��Pb����H����Cu��Hg��Ag��Pt��Au�����֪���Zn��Fe����H����Cu��
����⣺��1��Ŀǰ�������������ߵĽ���������
��2�����������������ˮ������ֱ�ӽӴ���Ҫ��֤��������ʱ������һ���μ��˷�Ӧ����Ҫ��������������ˮ��ͬ�����µķ�Ӧʵ�������ֻ��ˮ��û�������µ�ʵ�飮ʵ���������������ˮ��ͬ�����µķ�Ӧ��ʵ�������ֻ��ˮ�Ӵ��ķ�Ӧ��ʵ�������ֻ�������Ӵ��ķ�Ӧ��Ҫ��֤��������ʱ������һ���μ��˷�Ӧ����Ҫ��������������ˮ��ͬ�����µķ�Ӧʵ�������ֻ��ˮ��û�������µ�ʵ�飮���Ԣ٢ڣ�
��ѡA��
��3������������Ԫ�ص���������=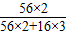 ×100%=70%��
×100%=70%��
1000t����Fe2O3����100%-20%=80%��80%�ij�����ʯ��������������Ϊ��1000t×80%=800t��
800t����������Ԫ�ص�����Ϊ��800t×70%=560t��
�ó������Ͽɲ���Fe98%�������������ǣ�560t÷98%=571.4t��
���571.4t��
��4������������������������Һ��Ӧ����ƫ�����ƺ�ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��Al2O3+2NaOH�T2NaAlO2+H2O��
��5���ɽ������˳��֪���Zn��Fe����H����Cu����������ͭ������п��ϡ����Ļ����Һ�У�����һ���������ۣ�ʹ֮��ַ�Ӧ������ʣ�࣬���ˣ�����Һ����ZnSO4��FeSO4��H2O����Һ�к��е����ǣ�Zn2+��Fe2+��SO42-��H2O�����������һ������Fe��Cu��
�ʴ�Ϊ��
��1������
��2��A��
��3��70%�� 571.4 t��
��4��Al2O3+2NaOH�T2NaAlO2+2H2O
��5��Fe��Cu��Zn2+��Fe2+��SO42-��H2O��
���������⿼���������������ʵ����ơ���ѧ����ʽ����д���������ļ����֪ʶ����һ�����Ѷȣ�
��2��Ҫ��֤��������ʱ������һ���μ��˷�Ӧ����Ҫ��������������ˮ��ͬ�����µķ�Ӧʵ�������ֻ��ˮ��û�������µ�ʵ�飮
��3���������ʵĻ�ѧʽ���Լ���ij��Ԫ�ص�����������ij�����ʵ�������
��4��������Ϣ����������������������Һ����Ӧ����ƫ�����ƣ�NaAlO2����ˮ��д����ѧ����ʽ��
��5����ǽ������˳��K��Ca��Na��Mg��Al��Zn��Fe��Sn��Pb����H����Cu��Hg��Ag��Pt��Au�����֪���Zn��Fe����H����Cu��
����⣺��1��Ŀǰ�������������ߵĽ���������
��2�����������������ˮ������ֱ�ӽӴ���Ҫ��֤��������ʱ������һ���μ��˷�Ӧ����Ҫ��������������ˮ��ͬ�����µķ�Ӧʵ�������ֻ��ˮ��û�������µ�ʵ�飮ʵ���������������ˮ��ͬ�����µķ�Ӧ��ʵ�������ֻ��ˮ�Ӵ��ķ�Ӧ��ʵ�������ֻ�������Ӵ��ķ�Ӧ��Ҫ��֤��������ʱ������һ���μ��˷�Ӧ����Ҫ��������������ˮ��ͬ�����µķ�Ӧʵ�������ֻ��ˮ��û�������µ�ʵ�飮���Ԣ٢ڣ�
��ѡA��
��3������������Ԫ�ص���������=
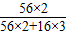 ×100%=70%��
×100%=70%��1000t����Fe2O3����100%-20%=80%��80%�ij�����ʯ��������������Ϊ��1000t×80%=800t��
800t����������Ԫ�ص�����Ϊ��800t×70%=560t��
�ó������Ͽɲ���Fe98%�������������ǣ�560t÷98%=571.4t��
���571.4t��
��4������������������������Һ��Ӧ����ƫ�����ƺ�ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��Al2O3+2NaOH�T2NaAlO2+H2O��
��5���ɽ������˳��֪���Zn��Fe����H����Cu����������ͭ������п��ϡ����Ļ����Һ�У�����һ���������ۣ�ʹ֮��ַ�Ӧ������ʣ�࣬���ˣ�����Һ����ZnSO4��FeSO4��H2O����Һ�к��е����ǣ�Zn2+��Fe2+��SO42-��H2O�����������һ������Fe��Cu��
�ʴ�Ϊ��
��1������
��2��A��
��3��70%�� 571.4 t��
��4��Al2O3+2NaOH�T2NaAlO2+2H2O
��5��Fe��Cu��Zn2+��Fe2+��SO42-��H2O��
���������⿼���������������ʵ����ơ���ѧ����ʽ����д���������ļ����֪ʶ����һ�����Ѷȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ